A group of scientists, including individuals from the College of Connecticut, has fostered a nanoparticle-based therapy that targets various offenders in glioblastoma, an especially forceful and lethal type of brain disease.
The outcomes of a cooperation among UConn and Yale College were distributed today in Science Advances.
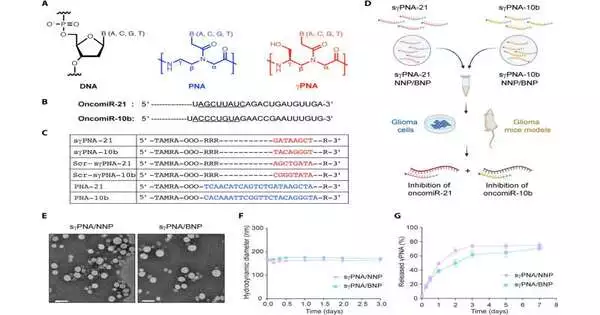
The new treatment utilizes bioadhesive nanoparticles that stick to the site of the growth and, afterward, leisurely deliver the blended peptide nucleic acids that they’re conveying. These peptide nucleic acids focus on specific microRNAs, that is, short strands of RNA that play a part in quality articulation. They are specifically targeting a type of overexpressed microRNA known as “oncomiRs,” which leads to the expansion of disease cells and the development of the growth.At the point when the peptide nucleic acids join the oncomiRs, they stop their cancer-advancing action.
The labs of teachers Raman Bahal of the UConn School of Pharmacy and Imprint Saltzman of Yale teamed up on the treatment framework. Unlike comparable efforts that target only one oncoprotein at a time, this treatment targets two, making its impact on disease cells more grounded. The test mice who received the treatment lived significantly longer than the control mice.
“It can strike down two targets at the same time, which has a surprisingly stronger consequence, as we showed with the enhanced survival results,”
Saltzman, a professor of biomedical engineering, chemical and environmental engineering, and physiology.
“It can thump down the two focuses simultaneously, which ends up having a strikingly more remarkable outcome, as we saw with the expanded endurance results,” says Saltzman, a teacher of biomedical design, compound and natural design, and physiology. “These outcomes are the best I’ve found at any point in this kind of forceful mind growth.”
One test in fostering the therapy was planning the counter-disease specialists, known as antimiRs, so two unique ones could fit in a solitary nanoparticle.
“We blended this multitude of mixtures and concocted the possibility that you don’t need to target each one in turn,” says Bahal, an academic partner in pharmaceutics. “Presently, we can ponder various Oncovir targets.”
For this work, the analysts designated the oncomiRs known as miR-10b and miR-21, which are both normal in glioblastoma. Future medicines, however, can be easily customized for specific patients. For example, in the event that a biopsy of a patient’s cancer creates a profile showing the expansion of various oncoproteins, the therapy could be suitably changed.
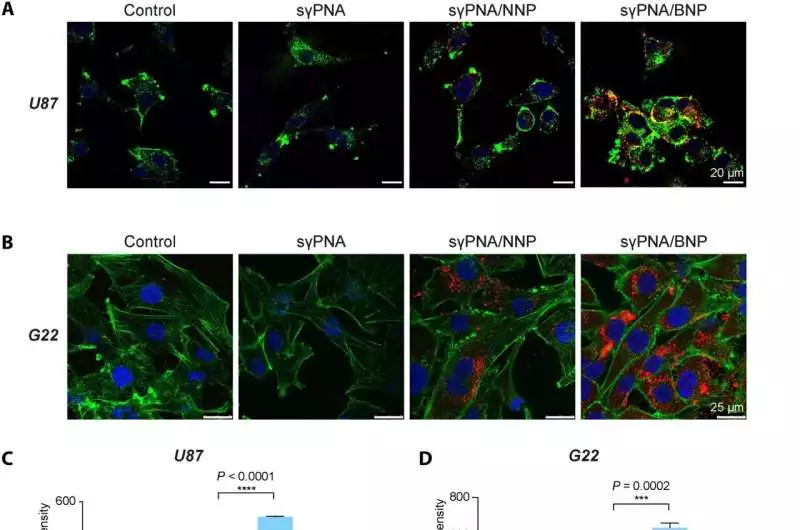
In various glioma cells, sPNA-interceded simultaneous knockdown of miR-21 and miR-10b.(A) A confocal, tiny picture of U87 cells treated with free sPNA or sPNA-stacked NPs Scale bars are 20 meters long.(B) Confocal, tiny pictures of G22 cells (patient-inferred GBM cells) treated with free sPNA or sPNA-stacked NPs. Scale bars are 25 meters long.PNAs were formed with TAMRA (red); F-actin was named with phalloidin (green), and the core was stained with Hoechst (blue). (C) Cell take-up in U87 cells examined by stream cytometry SD (n = 3) is used to communicate information.(D) Evaluation of cell take-up of sPNA in G22 cells through stream cytometry examination SD (n = 3) is used to communicate information.(E) Articulation examination of miR-10b and miR-21 levels in U87 cells after treatment with Scr-sPNA/NNP, Scr-sPNA/BNP, sPNA/NNP, sPNA/BNP, and free sPNA. (F) MiR-10b and miR-21 levels in G22 cells treated in vitro with sPNAs (sPNA-10b + sPNA-21) and BNPs containing sPNAs and scr-sPNAs.sPNA/BNPs are an actual combination of sPNA-21 BNP and sPNA-10b BNP. sPNA/NNPs are an actual combination of sPNA-21 NNP and sPNA-10b NNP. NNP shows PLA-HPG NP, and BNP demonstrates PLA-HPG-CHO NP.
Saltzman refers to the treatment as “a marriage of two innovations.”
“One is the bioadhesive nanoparticle innovation, which we had grown before, and marrying it to this peptide nucleic corrosive innovation that Raman has idealized,” he says.
Since the treatment is limited to the growth site, Bahal noticed that both the blended nucleic acids and the nanoparticles that convey them to the cancer site are non-harmful. The particles and specialists released by the treatment also remain at the growth site for around 40 days, which is critical to its success.Regular site-specific medications will generally wash away fairly quickly.
“These are high-restriction atoms that are versatile and viable all the while,” says Bahal. “It’s designated and remains there.” “Customary atoms have had many difficulties regarding harmfulness.”
Preferably, the conveyance framework would be applied as a feature of a larger treatment routine.
“We planned it to be an addition to what doctors do as of now,” Saltzman says. “They would perform a medical procedure, then they would mix our nanoparticles, and afterward, they would give chemotherapy as well as radiation in the manner that they typically do.” “We anticipate that this will result in a better outcome because the nanoparticle or hostility to microRNA is sharpening the cells to the chemotherapy and radiation treatment.”
The review’s different creators incorporate Shipra Malik, a previous alumni understudy in Bahal’s lab, and Vijender Singh, partner and head of the Computational Science Center at UConn.
“This study opens new roads for RNA-designated innovation in creating customized treatment for mental disease,” says Malik.
More information: Yazhe Wang et al, Anti-seed PNAs targeting multiple oncomiRs for brain tumor therapy, Science Advances (2023). DOI: 10.1126/sciadv.abq7459