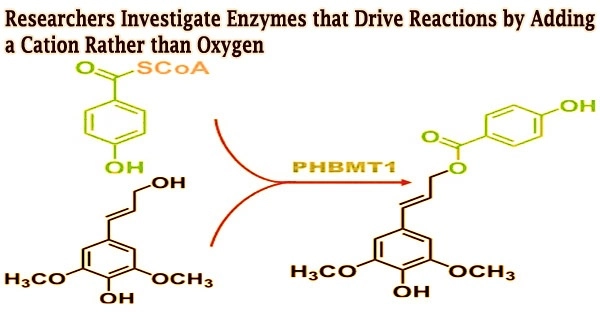
In order to determine whether these enzymes may be utilized to produce a variety of compounds, researchers from North Carolina State University and the University of Texas at Austin described the structure of a substrate-bound iron 2-oxoglutarate (Fe/2OG) enzyme. To find out how the enzyme would react with various substrates, they investigated its active site.
Additionally, they discovered that Fe/2OG enzymes probably use highly reactive cations to induce desaturation during catalysis rather than oxygen addition. The research could result in the creation of a variety of useful compounds using Fe/2OG enzymes.
The Fe/2OG family of enzymes are naturally occurring they are found in everything from bacteria to plants and animals.
As a result, these enzymes might be utilized to produce compounds like vinyl isonitriles, which have antibacterial capabilities, in a greener and more effective manner. However, little is known about the processes by which Fe/2OG enzymes produce these compounds.
“The endgame is to understand how the enzymes in this family create particular molecules, so that we can potentially piggyback on a natural process that current chemistry cannot replicate,” says Wei-chen Chang, associate professor of chemistry at North Carolina State University and co-corresponding author of a paper describing the work.
“So we looked at a couple of different enzymes within the Fe/2OG family to see how they performed different transformations using the same substrate, or molecule they bind to.”
Normally, the way Fe/2OG enzymes catalyze or create a new product happens like this: the enzyme binds to the substrate, a single oxygen atom from molecular oxygen (O2) is introduced into the substrate, and oxygen-addition drives the reaction. That process is called hydroxylation. But for these enzymes, the transformation or reaction isn’t driven by hydroxylation, but by a reactive cation that triggers the subsequent desaturations, where new bonds are introduced.
Wei-chen Chang
Researchers can identify additional substrates that the enzyme might employ by concentrating on how it binds to a single substrate; this method is more effective than experimental for identifying potential reactions and products.
The research team analyzed the architectures and end products of two Fe/2OG enzymes, PvcB and PlsnB. They were able to locate binding sites on both enzymes, but when they looked into how the enzyme carried out its modifications, they discovered something unexpected.
“Normally, the way Fe/2OG enzymes catalyze or create a new product happens like this: the enzyme binds to the substrate, a single oxygen atom from molecular oxygen (O2) is introduced into the substrate, and oxygen-addition drives the reaction,” Chang says.
“That process is called hydroxylation. But for these enzymes, the transformation or reaction isn’t driven by hydroxylation, but by a reactive cation that triggers the subsequent desaturations, where new bonds are introduced.”
Two Fe/2OG enzymes studied (PIsnB and PvcB) utilized fundamentally distinct desaturation to create different products from the same substrate.
“Now we know how these enzymes catalyze transformations and have found the binding sites, we have a foundation for determining what they can do in terms of reactions,” Chang says. “We can also recommend and predict the best substrates to use to get targeted products.”
The work appears in Nature Communications and was supported by the National Institutes of Health (GM127588, GM104896 and GM125882) and a Goodnight Early Career Innovator award.
Yan Zhang, professor of chemistry at the University of Texas at Austin, is co-corresponding author. NC State graduate student Tzu-Yu Chen and UT Austin graduate student Wantae Kim are co-first authors.