New primary data about a protein focus in disease medication could help the advancement of cutting-edge inhibitors. The protein, known as PARP1, recognizes DNA damage and sends a signal to repair it.PARP1 action is vital to numerous disease types, making it an alluring objective for medicine.
Clinical examinations have demonstrated the way that PARP1 inhibitors can be utilized as anti-growth therapies, working by upsetting DNA replication and repair to kill disease cells. As of late, scientists have started investigating whether PARP1 can likewise be utilized as an objective in medicines for different illnesses, including Alzheimer’s and Parkinson’s sickness, where the decrease in PARP1 hyperactivity can assist cells with making due.
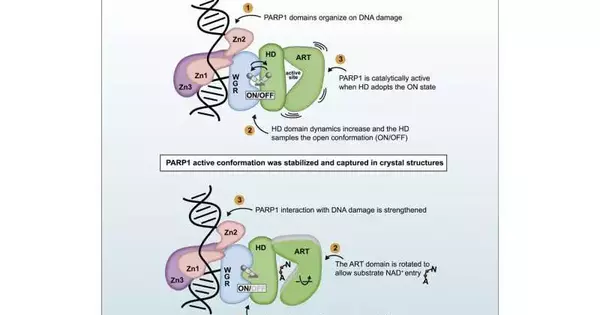
Interestingly, scientists from the Université de Montréal and the Institute of Cancer Research in the UK have caught a “preview” of PARP1 in the dynamic expression that it embraces following identifying DNA damage. The group’s new exploration, published in the journal Molecular Cell, assists how we might interpret how these proteins act and prepares for the up and coming age of PARP1 inhibitors.
The X-beam diffraction information that yielded these experiences was obtained by utilizing the CMCF beamline at the Canadian Light Source (CLS) at the University of Saskatchewan and the Advanced Light Source in the U.S.
The area of PARP1 that inhibitors assault is very portable, making it hard to completely grasp this moving objective, said Dr. John Pascal, a teacher with the Department of Biochemistry and Molecular Medicine at the Université de Montréal and an individual from this exploration group.
Certain inhibitors draw in the unique locales of PARP1 and can behave like a wrench stuck into a wheel or like a doorstop under an entryway, really assisting with locking PARP1 onto DNA harm, making sense of Pascal. “This method of hindrance could upgrade the system of eliminating malignant growth cells.” Conversely, inhibitors that stay away from the unique areas and come up short on “doorstop” impact could be more suitable for neurodegenerative illnesses, where cell protection is the objective instead of cell killing.
More information: Élise Rouleau-Turcotte et al, Captured snapshots of PARP1 in the active state reveal the mechanics of PARP1 allostery, Molecular Cell (2022). DOI: 10.1016/j.molcel.2022.06.011
Journal information: Molecular Cell