Pancreatic disease is a really harmful cancer in the stomach-related framework, and its five-year endurance rate is minimally over 10%. Metabolic changes are one of the signs of cancer cells. Oncogenic Kras-enacted pancreatic ductal adenocarcinoma (PDAC) cells rely intensely upon an unusual glutamine (Gln) catabolic pathway to support cell development.
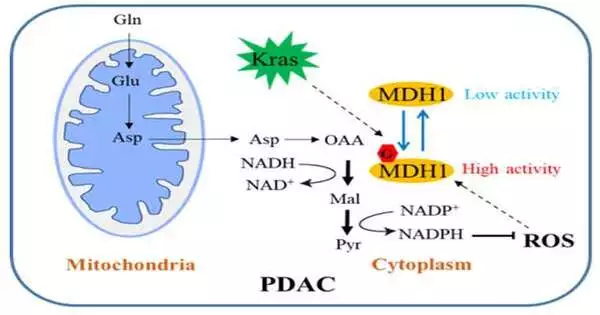
In the regular pathway, Gln is first changed over completely to aspartate (Asp), which is moved from the mitochondria into the cytosol where it is changed over successively by aspartate transaminase 1 (GOT1), MDH1 and malic protein 1 (ME1) to pyruvate and NADPH. This pathway is basic for PDAC cells to keep up with redox homeostasis and is expected for cell expansion and cancer development in vivo. Hence, a keen understanding of this administrative system might well open up another road for the clinical treatment of PDAC.
The examination group led by Prof. Zhou Ruhong and Prof. Yi Wen from the Zhejiang University College of Life Sciences distributed an article in the journal Nature Chemical Biology on July 25. This article reveals that O-GlcNAcylation contributes to pancreatic disease development by managing the metabolic action of ofmalate dehydrogenase 1 (MDH1). This finding has huge ramifications for the advancement of medications for pancreatic growth.
The group has led important exploration. According to their findings, the Kras mutation induces cell O-connected -N-acetylglucosamine (O-GlcNAc), a common type of protein glycosylation.Malate dehydrogenase 1 (MDH1), a vital protein in the glutamine catabolic pathway, is decidedly managed by O-GlcNAcylation on serine 189 (S189).
Sub-atomic elements recreations propose that S189 glycosylation on monomeric MDH1 upgrades the security of the substrate-restricting pocket and fortifies the substrate connections by filling them in as an atomic paste. Exhaustion of O-GlcNAcylation lessens MDH1 action, hinders glutamine digestion, sharpens PDAC cells to oxidative pressure, diminishes cell expansion, and represses cancer development in bare mice. Besides, O-GlcNAcylation levels of MDH1 are raised in clinical PDAC tests.
The identification of explicit pathways and proteins with novel reliance on O-GlcNAc is key for creating designated treatments. The concentrate by Prof. Zhou and Prof. Yi et al. reveals that MDH1 glycosylation is ready to direct the novel Gln metabolic pathway in PDAC, hence featuring the possibility to mediate in MDH1 glycosylation as a helpful system against PDAC.
More information: Qiang Zhu et al, O-GlcNAcylation promotes pancreatic tumor growth by regulating malate dehydrogenase 1, Nature Chemical Biology (2022). DOI: 10.1038/s41589-022-01085-5
Journal information: Nature Chemical Biology