An article written by doctoral student Md. Abdul Hamid and associate professor of MechSE Kyle Smith was recently published in the Journal of Power Sources.
“A bottom-up, multi-scale theory for transient mass transport of redox-active species through porous electrodes beyond the pseudo-steady limit” demonstrates that conventional theory approaches underestimate the power capacity of flow batteries like those used in the production of electricity from renewable energy sources.
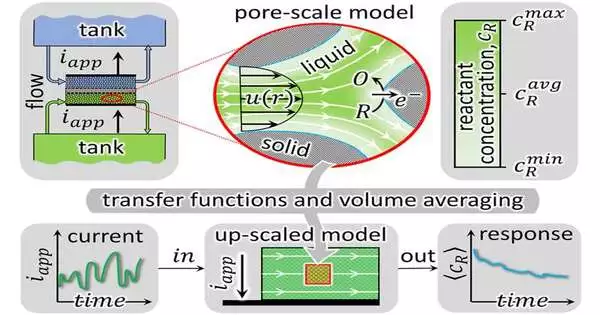
Smith described their theory, which proposes introducing certain frequency-dependent transfer functions to up-scale mass transport occurring in the microscopic pores of electrodes, as “a new means by which to understand how convection occurs inside of reactive porous media.” Although transfer functions are frequently utilized as mathematical tools in control theory, neither their application nor their derivation have ever been carried out in this manner before.
“These approaches have not previously been applied to the electrochemical systems that are the subject of our research.”
Associate Professor Kyle Smith.
Their publication was a long-awaited success because the two began developing their theory prior to the COVID-19 outbreak. They introduced a spectral Sherwood number, which is a type of transfer function, to extend the film law of mass transfer to transient conditions, which sheds new light on the well-known principles of mass and heat transfer. For convectional heat transfer, a spectral Nusselt number extends Newton’s law of cooling. In order to obtain flow batteries’ time-domain responses, the two developed the embedding of transfer functions into an upscaled model.
According to Smith, “We have uncovered a new understanding of certain non-dimensional parameters that are ubiquitous in convection heat and mass transfer. We have extended these concepts from their conventional application in time-invariant, or steady state, settings to transient settings in a way that takes into account shifts in the microscopic dynamics caused by transient cycling for the first time to our knowledge.”
This work also has significance for the chemical, civil, and petroleum engineering communities, which have investigated methods for comprehending mass transfer in other porous materials. ” “The electrochemical systems that are the subject of our work have not previously been addressed using their methods,” Smith stated. However, by employing a formulation that starts from the detailed microstructure and upscales its effects for use in macroscopic scale models, we have developed a relatively straightforward method for modeling these effects.”
In fact, the team’s theory shows that flow batteries can be used for short periods at a current higher than their limiting value, suggesting that lighter and cheaper batteries can be made for these cycling conditions.
Hamid stated, “These findings not only provide guidelines toward more efficient designs, operating schemes, and materials but also provide a better modeling approach for accurate prediction of an existing flow battery’s rate capability.”
Using electrochemistry, Smith and Hamid intend to apply their theory to various microstructures for porous electrodes in energy and environmental devices. Extending the theory beyond the range of conditions demonstrated in their publication is also one of their next steps.
More information: Md Abdul Hamid et al, A bottom-up, multi-scale theory for transient mass transport of redox-active species through porous electrodes beyond the pseudo-steady limit, Journal of Power Sources (2023). DOI: 10.1016/j.jpowsour.2023.232756