Researchers keep on growing the mechanical outskirts of CRISPR, alongside its huge potential, in regions ranging from human wellbeing to worldwide food supplies. Such is the case with CRISPR-based quality drives, a hereditary altering device designed to influence how hereditary components are passed down from one generation to the next.
Quality repellents intended for mosquitoes can possibly check the spread of malarial diseases that cause countless deaths every year, yet security issues have been raised on the grounds that such repellents can spread rapidly and rule whole populations. Researchers have investigated the standards overseeing the spread of quality drive components in designated populations, for example, mosquitoes, by testing various mixes of parts that comprise the drive device. They have found, nonetheless, that there’s something else to investigate and that key inquiries remain.
In the diary Nature Correspondences, College of California San Diego scientists led by previous postdoctoral researcher Gerard Terradas, along with postdoctoral researcher Zhiqian Li and teacher Ethan Casket, in close cooperation with UC Berkeley graduate understudy Jared Bennett and academic partner John Marshall, depict the improvement of another framework for testing and creating quality drives in the lab and securely changing them over into devices for possible true applications.
“These examinations […] engage new designs of quality drive frameworks while giving significant data in regards to how to survey and dissect key connections between their most significant moving parts,” said Coffin, an employee in the School of Organic Sciences, Branch of Cell and Formative Science.
CRISPR-based quality drives highlight a protein called a Cas9 endonuclease and an aid RNA particle that unite to guide DNA slices to explicit locales in the genome where new hereditary components can be embedded. As the DNA fixes these cuts, the new hereditary components are replicated, starting with one chromosome and moving onto the next, bringing about a posterity that surpasses the standard 50-50 legacy, leaning more toward the recently embedded hereditary components.
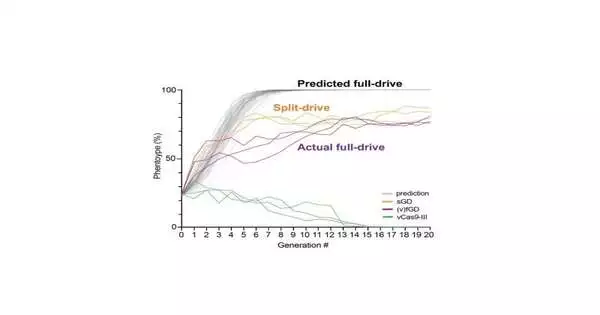
This realistic representation depicts the numerically anticipated frequencies north of a few ages of a full-quality drive (dim), contrasted and the lab experiments with a “hacked” split-quality drive (orange) and a full-quality drive (purple).
Quality drives come in two “flavors.” Full-quality drives (fGDs) convey both the Cas9 and guide RNA parts in a connected unitary bundle. Conversely, split drives (sGDs) consist of two hereditary components that independently convey the Cas9 and guide RNA parts and are embedded at various locales in the genome. Divide drives are viewed as more secure than fGDs since it is feasible to control and test the parts conveyed by every one of the components independently or under conditions where they slowly enhance the recurrence of the gRNA part. Scientists plan for the two components to ultimately reconnect to convey the impacts of a full-quality drive.
On account of killing jungle fever, full-quality drives have consumed extensive energy because of their true capacity as vehicles to move components that end the transmission of malarial parasites that cause disease. Yet, fGDs have likewise raised concerns because of their capability to spread and possibly alter the hereditary makeup of whole mosquito populations quickly. Exploring various avenues for FGDs necessitates high-security boundaries and constraints to prevent accidental breakage of bugs conveying such crashes out of the dark climate.
This isn’t true with split-quality drives. Because the key components are isolated, sGDs carry significantly less risk of accidental spread, and analysts have significantly more control over their protected control.explores different avenues regarding how sGDs can be led in customary lab offices, thereby permitting considerably more adaptability for testing their true capacity.
Researchers have been tested, nonetheless, in creating frameworks that really convert sGDs into completely working fGDs. One test of the current change of sGD frameworks into fGD frameworks is that they depend on two separate hereditary parts, each of which should show effective drive properties.
Presently, UC San Diego researchers who have as of late spearheaded quality drive improvement and related innovations have made an adaptable hereditary “hacking” framework for changing over sGDs into fGDs. Working in organic product flies, the scientists fostered a clever hereditary system that utilizes a uniquely planned guide RNA conveyed by the Cas9 part of the sGD. This hacking device cuts the duplicating part of the sGD and triggers a hereditary trade, or “recombination occasion,” that embeds the Cas9 into the component conveying the aid RNA, bringing about the making of a completely working fGD.

Four fly boards: Transgenic organic product flies used in quality drive research contain three fluorescent transgenes (green for GFP, green for DsRed, and cyan for CFP).
“First and foremost, and most importantly, the review provides evidence of rule to the deft hereditary change of a sGD into a fGD, which ought to enormously support the testing and advancement of new, improved quality drive frameworks,” said Terradas, who is now based at Penn State College.
When the analysts fostered their new sGD-to-fGD hacking framework, a few amazing outcomes started to arise. The recently hacked fGD spread through populations of flies in confinement tests, true to form. In any case, the rate at which it spread was suddenly slower than models had anticipated for a customary fGD.
Research partners Bennett and Marshall developed a numerical model that provided clarification. Their model uncovered that during the hacking change, fGDs imposed a more prominent wellness cost on individual flies than sGDs alone. This health cost, which emerges when the drive component duplicates itself, vanished after following up on all potential target chromosomes in the population.
“The review uncovers unforeseen intricacies in how quality drive parts cooperate, revealing that one can’t just expect how separate parts might connect when united,” said Bennett.
The paper’s full creator list incorporates Gerard Terradas, Jared Bennett, Zhiqian Li, John Marshall, and Ethan Coffin.
More information: Gerard Terradas et al, Genetic conversion of a split-drive into a full-drive element, Nature Communications (2023). DOI: 10.1038/s41467-022-35044-4





