An examination group has uncovered how zinc carrier buildings control zinc particle (Zn2+) focuses in various regions of the Golgi device and uncovered that this system finely tunes the chaperone protein ERp44.
Zinc homeostasis is essential for avoiding fatal diseases like diabetes, cancer, growth failure, and immunodeficiency, and the key chemical and cellular biological mechanisms at work behind it were revealed in the findings, which were published in the journal Nature Communications on May 9, 2023.
Zinc is an essential mineral for our health as a trace element. Nearly 10% of the human proteome binds Zn2+ for structural maturation and function, which is essential for enzyme catalysis, protein folding, DNA binding, and regulating gene expression.
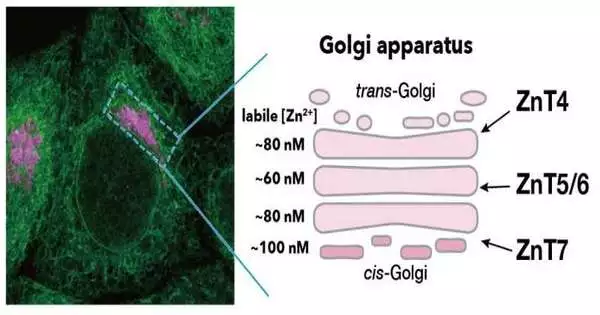
“We discovered that these ZnT complexes regulate the Zn2+ concentrations in the various Golgi compartments, specifically the cis, medial, and trans-Golgi cisternae, using a combination of chemical biology and cell biology techniques.”
Kenji Inaba, a corresponding author of the study .
The endoplasmic reticulum (ER), a complex membrane network of tubules, is where secretory proteins like hormones, immunoglobulins, and blood clotting factors are made and folded. After that, they are moved to the organelle known as the Golgi apparatus, which is made up of a number of flattened sacs called cisternae and matures there. This organelle sorts and processes proteins before directing them to a specific location. Chaperone proteins are crucial for keeping up with protein homeostasis and forestalling the development of misfolded or total proteins in these organelles.
In the early secretory pathway that includes the ER and the Golgi, previous research by the group demonstrated that Zn2+ in the Golgi apparatus is crucial to protein quality control. The ER-Golgi cycling chaperone protein ERp44 controls this system.
Three ZnT complexes exist in the Golgi apparatus: ZnT7, ZnT4, and Znt5/6. However, the mechanisms by which the Golgi apparatus maintains Zn2+ homeostasis have remained a mystery up until this point.
“We revealed that these ZnT complexes regulate the Zn2+ concentrations in the different Golgi compartments, namely cis, medial, and trans-Golgi cisternae,” says Kenji Inaba, a corresponding author of the study and professor at Tohoku University’s Institute of Multidisciplinary Research for Advanced Materials Sciences. “Using chemical biology and cell biology approaches together, we revealed that these ZnT complexes regulate the Zn2+ concentrations.” We additionally further clarified the intracellular vehicle, restriction, and capability of ERp44 constrained by ZnT edifices.”
To prevent abnormal secretion, ERp44 binds to immature secretory proteins at the Golgi apparatus. Heart failure and hypotension were observed in mice whose ERp44 expression was suppressed in previous research.
Additionally, numerous diseases, such as cancer cell metastasis and hypophosphatasia, are associated with a number of secretory zinc enzymes. For enzymatic activity, these enzymes require Zn2+, which they acquire from the Golgi-resident ZnT complexes. Male mice with ZnT5 smothered have encountered demise brought about by arrhythmias, so there is conceivable importance of Zn2+ homeostasis to cardiovascular illness.
“Our discoveries will assist us with understanding the system by which disturbances of Zn2+ homeostasis in the early secretory pathway prompt the advancement of neurotic circumstances,” adds Inaba.
The group is confident that the techniques utilized in their review can illustrate the components fundamental to the support of intracellular Zn2+ homeostasis, suggesting future examinations that can gauge Zn2+ in different organelles, for example, the mitochondria and core.
More information: Yuta Amagai et al, Zinc homeostasis governed by Golgi-resident ZnT family members regulates ERp44-mediated proteostasis at the ER-Golgi interface, Nature Communications (2023). DOI: 10.1038/s41467-023-38397-6