Methane, chiefly from petroleum gas, shale gas, and methane hydrate, is perhaps the most expensive non-renewable energy source. Nonetheless, it remains an extraordinary test to understand the specific valorization of methane under gentle circumstances because of the innately low polarizability and high separation energy of the C-H bond in CH4 as well as the higher reactivity of target oxygenates.
As of late, an exploration team led by Prof. Wang Xiaodong and Prof. Lin Jian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), as a team with Prof. Zhu Chun from Guizhou University, has created UiO-66 metal-natural systems (MOFs) impetuses with tunable electronic properties, which could improve the specific oxidation of methane.
This study was published in Angewandte Chemie International Edition on June 29.
On the supported metal species, over-oxidation of target items and metal locales draining are normally undeniable.In the interim, the organized metal-oxo species on mass metal oxides or systems are sub-par reactivity, and they simply act as a help or co-impetus to upgrade the CH4 change.
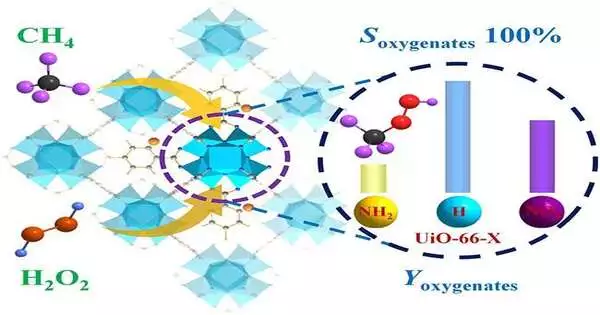
To address these issues, the researchers used the sole UiO-66 MOF impetuses changed by different ligands (NH2-BDC, H2BDC, and NO2-BDC) to directly convert CH4 to oxygenates with 100 percent selectivity by utilizing H2O2 as an oxidant under mild conditions.
The Zr-oxo hubs with these modifiers showed different electronic properties that impacted the mooring of OH species to frame viable Zroxo-OH locales, which could advance the actuation of the C-H obligation of CH4 with the least energy boundary over UiO-66-H.
More information: Geqian Fang et al, Zirconium‐oxo Nodes of MOFs with Tunable Electronic Properties Provide Effective ⋅OH Species for Enhanced Methane Hydroxylation, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202205077
Journal information: Angewandte Chemie International Edition