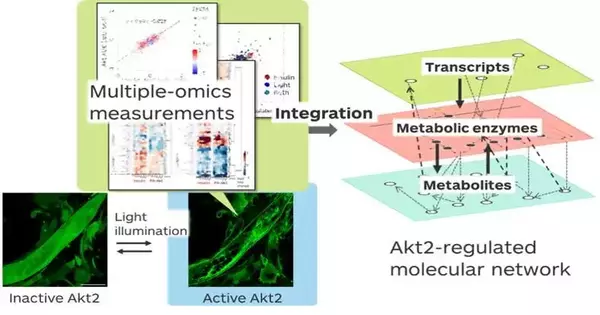
Takeaki Ozawa and his team from the University of Tokyo have revealed the metabolic reactions that take place after activating an enzyme called Akt2. In doing so, they reveal the inner workings of insulin-regulated metabolism. The findings pave the way for Akt2-targeting therapeutics for diabetes and metabolic disorders.
It takes energy to do anything—even to exist. You can metabolize food to convert glucose into energy, thanks to many cascades of molecular reactions within your cells. As soon as you eat, your pancreas secretes insulin, which starts various metabolic processes. As if it were a game of molecular relay, insulin binding to its receptors triggers a communication chain via the molecular reactions mediated by enzymes called kinases. Akt2 is one such kinase involved in insulin-regulated cellular metabolic processes.
“Cellular metabolism includes many molecular pathways that regulate nutrient storage and energy production—all influenced or controlled by insulin.” We know many of the key players involved in insulin pathways. “But we are now interested in how these players play a role as individuals,” Ozawa said when asked about their motivation to study Akt2.
“Cellular metabolism involves numerous molecular pathways that regulate nutrition storage and energy production, all of which are impacted or controlled by insulin. Many of the essential participants in insulin pathways are well known. But, we are now interested in how these guys act as individuals.”
Takeaki Ozawa and his team from the University of Tokyo.
But it is not an easy task. Cellular metabolism is like a busy market with RNAs, proteins, and metabolites working simultaneously. That makes studying a specific biomolecule in the process challenging. And if even one biomolecule doesn’t function normally, it leads to metabolic disorders such as diabetes. So, Ozawa and his team set out to tackle this challenging problem.
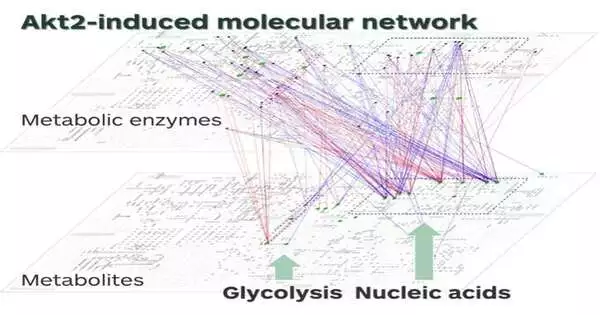
Akt2’s role in glycolysis and nucleotide metabolism stands out from the molecular network.
They used a new analytical method called “transomics” analysis paired with “optogenetics” technology. Transomics analysis combines large-scale data of biomolecules involved in the metabolic process: proteins (proteomics), expressed genes or RNA transcripts (transcriptomics), and metabolites (metabolomics). Optogenetics technology allowed the researchers to specifically activate Akt2 by shining light onto light-sensitive Akt2 molecules inside cells. When they turn the light on, all the Akt2 molecules are assembled at the cell membrane. Turn off the light; Akt2 is deactivated.
To understand the consequences of Akt2 activation, the researchers activated Akt2 in mouse skeletal muscle cells. Then they collected large-scale data on the biomolecules that were produced or degraded soon after. The transomics analysis revealed the molecular network triggered by Akt2 activation.
To their surprise, Akt2 uses different regulatory mechanisms compared to insulin. The Akt2-regulated network included 9 genes, 56 metabolic enzymes, and 23 metabolites. But the insulin-regulated network included 32 genes, 43 metabolic enzymes, and 18 metabolites. In some metabolic reactions, Akt2 acts alone; in others, it acts with other enzymes. Notably, Akt2 plays a vital role in initiating glycolysis, which involves breaking down glucose to produce energy, and nucleotide metabolism, which involves synthesizing or breaking down DNA and RNA.
“These results can contribute to elucidating the mechanisms of disease onset caused by mutations in Akt2 function,” Ozawa said. “They can also help the development of drugs targeting Akt2.” The analytical framework used in this study is applicable to other biomolecules too. So, this approach is versatile for analyzing the function of a specific enzyme inside a cell.
More information: Optogenetic decoding of Akt2-regulated metabolic signaling pathways in skeletal muscle cells using transomics analysis, Science Signaling (2023). DOI: 10.1126/scisignal.abn0782