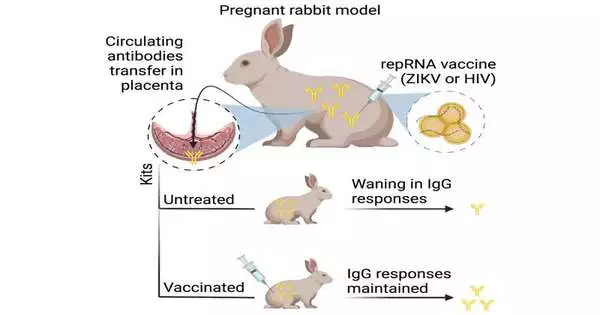
According to a study published in the journal Molecular Therapy, newly developed mRNA vaccines against HIV-1 and Zika virus generated robust antibody responses that were passed on from pregnant rabbits to their offspring. The findings, as noted by the authors, support the development of their vaccine platform, LION/repRNA, for use in maternal and neonatal settings to prevent the transmission of pathogens from mother to child in both animals and humans.
The development of mRNA vaccines for other infectious diseases will be sparked by the recent success of mRNA vaccines against the COVID-19 pandemic. mRNA vaccines have been approved by the Food and Drug Administration of the United States for use in children aged six months and older, and preliminary research on pregnant women has revealed no obvious adverse effects.
Senior author Amit Khandhar, a material scientist at HDT Bio Corp., says, “Preventing mother-to-child transmission is a major goal for reducing disease burden in newborns.” “With mRNA vaccines attracting global attention, there is a need to evaluate their safety and immunogenicity in preclinical models that inform maternal and childhood vaccination.”
“A primary goal for reducing illness burden in infants is to prevent mother-to-child transmission. With mRNA vaccines gaining interest around the world, there is a need to investigate their safety and immunogenicity in preclinical models that influence maternal and pediatric immunization.”
Senior author Amit Khandhar, a material scientist at HDT Bio Corp.
Self-amplifying replicon (repRNA) vaccines were evaluated by Khandhar, Herman Staats of Duke University School of Medicine, and Noah Sather of Seattle Children’s Research Institute. Using Zika virus and HIV-1 as model diseases, the researchers administered the vaccines to pregnant rabbits using their clinical-stage LION nanoparticle formulation. After being passed from mother to child, these two pathogens play a significant role in the transmission of infections to newborns.
The repRNA vaccines contain viral enzymes that outperform non-replicating mRNA in terms of dosing and manufacturing because they increase the expression of a particular gene by 10 to 100 times.
In contrast to lipid nanoparticle formulations, which encapsulate RNA, the exclusive LION delivery technology is a stable oil-in-water nanoparticle emulsion that electrostatically binds and safeguards nucleic acids. LION has plug-and-play functionality because it is stored independently of repRNA. This makes it possible to quickly evaluate new repRNA vaccine constructs, like those that were recently developed to deal with new SARS-CoV-2 variants.
The findings demonstrated that immunization with repRNA at a relatively high dose was well tolerated and did not have any negative effects on litter size. “While the strong correlation in both the magnitude and quality of antibody levels between mothers and newborns suggests that the antibodies detected in kits were likely acquired passively from mothers, we cannot completely rule out the possibility that the vaccine administered to mothers may itself distribute to kits and actively induce antibody responses,” Khandhar says. The LION and repRNA vaccines also triggered robust antigen-specific antibody responses in pregnant adult rabbits.
Additionally, the researchers discovered that subsequent vaccination of newborns maintained elevated antibody levels in comparison to no vaccination and that the timing of the maternal vaccination was crucial for maximising antibody transfer. Active immunization of newborns may be necessary for maintaining overall antibody responses in infants after birth, in addition to optimal timing of maternal vaccinations. According to the authors, additional research is required to ascertain whether RNA-based maternal vaccines can provide protection against infection transmitted from mother to child.
According to Khandhar, “for example, the immunization intervals we used were not optimized and will likely not translate to humans due to differences in the gestational periods between rabbits and humans.” In order to maximize passive antibody transfer to newborns, additional research will be required to test boosting intervals and antibody response durability. In conclusion, extra examinations intended to gauge wellbeing signals in maternal and neonatal models will be required prior to progressing to clinical assessment.”
More information: Amit P. Khandhar et al, Evaluation of repRNA vaccine for induction and in utero transfer of maternal antibodies in a pregnant rabbit model, Molecular Therapy (2023). DOI: 10.1016/j.ymthe.2023.02.022