A completely new way to deal with monoclonal immunization treatment shows that focusing on the more hereditarily stable inner protein of the SARS-CoV-2 infection as opposed to the surface spike protein can likewise clear SARS-CoV-2, reports a review from Northwestern Medication and the College of Illinois at Chicago (UIC), distributed in the Diary of Clinical Examination.
Some monoclonal immunizer medicines have quit working on the grounds that the spike viral protein goes through high rates of change, delivering a few viral variations impervious to current neutralizer treatments. The clever methodology could give another weapon in medicine that could save adequacy as the spike protein changes.
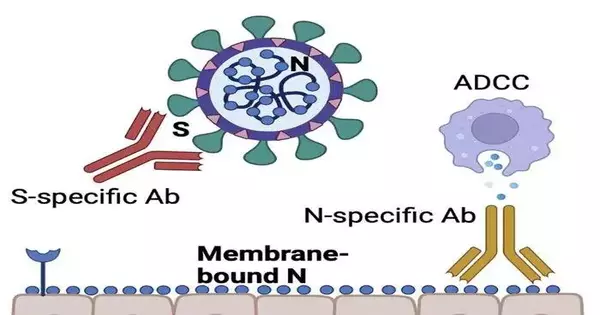
This is whenever helpful monoclonal antibodies first designate an inner instead of a surface protein.
“These outcomes may likewise add to the improvement of joined immunizer treatments for SARS-CoV-2 as well as other viral illnesses, for example, HIV, by focusing on unusual viral proteins that are not normally designated by monoclonal neutralizer treatments,” said co-relationship creator Pablo Penaloza-MacMaster, Ph.D., aide professor of Microbial Science and Immunology.
“These findings may also aid in the development of combined antibody therapies for SARS-CoV-2 and other viral diseases such as HIV by targeting unconventional viral proteins that are not typically targeted by monoclonal antibody therapies,”
Pablo Penaloza-MacMaster, Ph.D., assistant professor of Microbiology-Immunology.
The main creator is Tanushree Dangi, Ph.D., an examination partner in the Penaloza-MacMaster lab.
How this approach is unique
All the monoclonal immunizer treatments for SARS-CoV-2 depend just on a superficial level of spike protein since this viral protein intercedes into the cell.
“It resembles a key and lock framework to enter the cell,” Penaloza-MacMaster said. “You need to have antibodies that forestall the key — the spike — from entering the lock. Yet, the key has been changing as the infection transforms, and some immunization medicines are, as of now, not viable at impeding the key from opening the lock. The ongoing immunizer treatments could lose adequacy later on. We found out if focusing on the inner piece of the infection by an immunizer treatment would give security.
The nucleocapsid protein is available inside SARS-CoV-2, yet when a cell gets tainted, the nucleocapsid protein is presented to the cell surface. The nucleocapsid is among the most plentiful proteins in SARS-CoV-2, making it a great objective for antibodies that can then enroll cells into the safe framework.
Hello, here’s an interloper!’
The tainted cells will be shrouded in nucleocapsid, and afterward the antibodies will bind to the nucleocapsid on the outer layer of the cells, which will draw in safe cells to kill the disease, Penaloza-MacMaster said.
“Antibodies not just keep the infection from entering the phone, yet they can likewise behave like a bull horn that calls cells of the safe framework, advising them to kill the tainted cell,” Penaloza-MacMaster said. “It resembles the immunizer is denoting the tainted cells for obliteration by calling, ‘Hello, here’s an interloper!'”
In the review, researchers tainted mice with the coronavirus through their noses, the same way people contract it. Then, one group of mice was treated with the monoclonal antibodies focusing on the nucleocapsid protein. Another gathering got control antibodies, not focusing on this protein.
Agents then analyzed the lungs to see how much infection and irritation there was. The mice that got the nucleocapsid immunizer had altogether less infection and less irritation in their lungs. They likewise had fewer fiery cytokines that are known to demolish the coronavirus when they are created at undeniable levels.
“These outcomes warrant the clinical assessment of monoclonal antibodies that target other flighty proteins of the infection and not just the spike protein,” Penaloza-MacMaster said.
More information: Tanushree Dangi et al, Improved control of SARS-CoV-2 by treatment with nucleocapsid-specific monoclonal antibody, Journal of Clinical Investigation (2022). DOI: 10.1172/JCI162282
Journal information: Journal of Clinical Investigation