Scientists at Tufts College have fostered a strategy to make silk-based materials that won’t adhere to water, or nearly anything else containing water besides. Truth be told, the changed silk, which can be shaped into structures like plastic or covered onto surfaces as a film, has non-stick properties that outperform those of nonstick surfaces commonly utilized on cookware, and it could see applications that reach out into an extensive variety of consumer items as well as medication.
Silk is a characteristic fiber turned by moths and has been utilized for millennia to make solid and fine textures, as well as careful stitches to contain wounds.
Researchers have recently discovered how to separate the strands to their essential protein component — silk fibroin — and reconstitute them into gels, films, wipes, and various structures to make everything from implantable muscular screws to material inks that change variety due to body science.
“What makes silk such a novel material is that, besides the fact that it can take on many structures and shapes, one can undoubtedly change its properties by synthetically changing the silk fibroin,” said Krishna Kumar, Robinson Teacher of Science at Tufts.
“What makes silk such a unique material is that it can not only take on a vast range of forms and shapes, but its qualities can also be easily changed by chemically changing the silk fibroin,”
Krishna Kumar, Robinson Professor of Chemistry at Tufts.
“To make muscular screws that are consumed by the body at various rates utilizing silk fibroin, we alter the science,” he said. “To make a blood sensor that identifies oxygen, or glucose, or other blood parts, we changed the science. “In this review, we changed silk fibroin to repel water, and we can do it in a way that can ‘tune’ the material to be pretty much water repellant.”
The development was accounted for in the diary ChemBioChem.
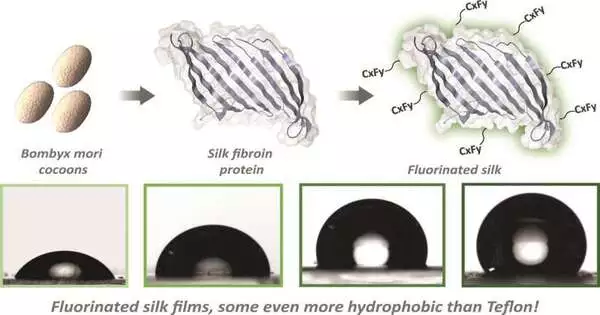
Transforming silk into a water repellant material involves covering the outer layer of the silk fibroin with short compound chains containing carbon and fluorine, called perfluorocarbons. These chains are truly steady and don’t respond to different synthetics, nor do they connect with proteins and other natural synthetics in the body.
While the normal surface of the silk protein behaves like a magnet to water, with adversely and decidedly charged branches on the silk drawing in water, a silk protein covered with perfluorocarbons passes on little for the water to take hold of.
Perfluorocarbons even oppose fascination brought about by different powers that commonly unite atoms. Changing the number and length of perfluorocarbon chains on the silk protein can change how “unsticky” it acts. Luke Davis, Aide Teacher of Science, laid out the degree of fluorine expected on the outer layer of silk to show a nonstick way of behaving.
The compound blend is finished under gentle circumstances, so dissimilar to the creation of other non-stick substances, the assembling system could be more secure, both for laborers and the climate More secure assembling and an inexhaustible organic wellspring of material confirm two boxes for manageability.
The Tufts scientists estimated the nonstick property by seeing how water dots up on the outer layer of the material—like how water rolls off a waxed vehicle. Truth be told, on nonstick silk formed into bars utilizing the most elevated level of perfluorocarbons, the water moves up into drops that are adjusted much more tightly than they do on Teflon.
Water rolls off the nonstick silk, but any substance containing water could include various food sources, blood, cells, and tissues.Although not tried in this review, perfluorinated materials are referred to as repulsive oils too.
Changes in clinical gadgets to forestall adverse connections with water and other biologics can possibly save strength and honesty however long they are required, “made sense of by Julia Wellspring, graduate understudy in Kumar’s lab and co-creator of the paper. “Silk is now generally idle to the safety framework, so tuning its capacity to repulse cells or different substances could make it much more helpful.”
The upsides of profoundly nonstick surfaces work out positively past clinical applications. While there is concern in regards to synthetics retained in the body from monetarily accessible nonstick coatings, silk-based non-stick surfaces might offer an elective choice which can be investigated for its relative security.
One could also imagine car windshields where water simply rolls off without the use of wipers; coatings on metals to help prevent rust; or textures to make them easier to clean.
“The achievement we had with altering silk to repulse water expands our triumphs with synthetically changing silk for different functionalities—for example, the capacity to change tone, lead electrical charge, or endure or debase in a natural climate,” said David Kaplan, Harsh Family Teacher of Designing at Tufts. “As a protein, silk lends itself well to measured science—the capacity to ‘connect’ different useful parts on a characteristic platform.”
More information: Julia N. Fountain et al, Towards Non‐stick Silk: Tuning the Hydrophobicity of Silk Fibroin Protein, ChemBioChem (2022). DOI: 10.1002/cbic.202200429
Journal information: ChemBioChem