Researchers are saddled with a better approach to transforming disease cells into strong, hostile malignant growth specialists. In the most recent work from the lab of Khalid Shah, MS, Ph.D., at Brigham and Women’s Clinic, an establishing individual from the Mass General Brigham medical care framework, examiners have fostered another phone treatment way to deal with laid-out growths and prompt long-haul resistance, preparing the safe framework so it can keep disease from repeating. The researchers tested their double activity, disease-killing immunization, in a high-level mouse model of the lethal brain malignant growth glioblastoma, and the results were promising.Discoveries are distributed in Science Translational Medicine.
“Our group has sought after a basic thought: to take disease cells and change them into malignant growth executors and immunizations,” said relating creator Khalid Shah, MS, Ph.D., head of the Middle for Immature Microorganism and Translational Immunotherapy (CSTI) and the bad habit seat of exploration in the Branch of Neurosurgery at the Brigham and staff at the Harvard Clinical School and Harvard Undifferentiated Organism Foundation (HSCI). “Utilizing quality design, we are reusing disease cells to foster a system that eliminates growth cells and invigorates the safe framework to both obliterate essential cancers and forestall disease.”
“Our team explored a basic idea: to turn cancer cells into cancer killers and vaccinations. We are repurposing cancer cells using gene engineering to create a treatment that kills tumor cells while also stimulating the immune system in order to remove primary tumors and prevent cancer.”
Khalid Shah, MS, Ph.D., director of the Center for Stem Cell and Translational Immunotherapy (CSTI)
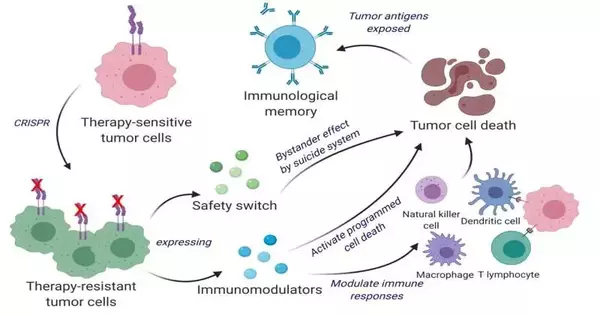
Disease immunizations are a functional area of exploration for some labs, yet the methodology that Shah and his partners have taken is particular. Rather than utilizing inactivated growth cells, the group reuses living cancer cells, which have a strange element. Like homing pigeons getting back to their perches, living growth cells will traverse the mind to get back to the site of their kindred cancer cells. Exploiting this novel property, Shah’s group designed living growth cells utilizing the quality-altering device CRISPR-Cas9 and reused them to deliver cancer cell specialists. Likewise, the designed growth cells were intended to communicate factors that would make them simple for the safe framework to detect, tag, and recall, preparing the framework for a drawn-out enemy of cancer reaction.
The group tried their reused CRISPR-improved technology and figured out helpful cancer cells (ThTC) in various mouse strains, including the one that bore bone marrow, liver, and thymus cells from people, copying the human safe microenvironment. Shah’s group likewise fabricated a two-layered security switch for the disease cell, which, when enacted, kills THCs if necessary. This double-activity cell treatment was protected, relevant, and solid in these models, proposing a guide toward treatment. While additional testing and advancement is required, Shah’s group explicitly picked this model and utilized human cells to smooth the way of deciphering their discoveries in patient settings.
“All through all of the work that we do in the middle, in any event, when it is profoundly specialized, we never fail to focus on the patient,” said Shah. “We want to adopt a creative yet translatable strategy so we can foster a helpful, disease-killing immunization that eventually will have an enduring effect on medication.” Shah and his partners note that this remedial system is relevant to a more extensive scope of strong growths and that further examinations of its applications are justified.
More information: Kok-Siong Chen et al, Bifunctional cancer cell-based vaccine concomitantly drives direct tumor killing and antitumor immunity, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abo4778. www.science.org/doi/10.1126/scitranslmed.abo4778
Journal information: Science Translational Medicine