A dangerous atmospheric deviation has been credited to the sharp expansion in heat-catching ozone-depleting substance discharges, specifically CO2 outflows. A promising approach to reducing emissions is carbon capture technology, such as the utilization of adsorbents to capture and store CO2 from the surrounding air.
Traditionally, liquid sorbents have been used to capture carbon, but their equipment corrosion, high cost, and high energy requirements for regeneration make them unsuitable. Solid porous materials for CO2 adsorption, in which CO2 atoms adhere to the surface of the solid material, are being investigated as a means of overcoming these limitations.
In his carbon catch research, Academic partner Wu Ping of the Singapore College of Innovation and Planning (SUTD) went to mica, a modest and plentiful mud mineral with different applications.
Mica forms alumina silicate sheets that are connected by ionic bonds formed by potassium cations between the layers. Mica, on the other hand, has a complex structure that makes it difficult to separate it into layers to create two-dimensional (2D) nanosheets that are good for CO2 capture. In addition, previous research’s methods required lengthy reaction times and a lot of energy.
“Building on our recent mechanochemistry breakthroughs, we have creatively combined microwave chemistry and solvothermal mechanochemistry. We were able to transform the energy from microwaves and solvothermal processes into strain energy inside solid-liquid-gas interfaces, allowing the creation of exfoliated mica (eMica) nanosheets. These activities cause quick exfoliation and a substantially shorter reaction time.”
Associate Professor Wu Ping of the Singapore University of Technology and Design (SUTD).
Assoc. Prof. Wu and his SUTD team worked with researchers from the Agency for Science, Technology, and Research (A*STAR) to develop an effective method for producing 2D mica nanosheets. They distributed their examination paper, “Effective combination of 2D mica nanosheets by solvothermal and microwave-aided procedures for CO2 catch applications,” in Materials.
“Expanding upon our new leaps forward in mechanochemistry, we have creatively consolidated the strategies of microwave science and solvothermal mechanochemistry. We were able to convert the energy from microwaves and solvothermal processes into strain energy at solid-liquid-gas interfaces, making it easier to make exfoliated mica (eMica) nanosheets. Assoc. Prof. Wu explained, “These actions result in rapid exfoliation and significantly reduced reaction time.”
In a closed reaction vessel, the research team mixed natural mica with potassium hydroxide in a polar solvent. This response was then warmed in a microwave, moving energy to the microwave-engrossing polar dissolvable and the reactants. The mica was exfoliated quickly and with a significantly reduced reaction time thanks to the vessel’s own pressure. The microwave-treated mica was then sonicated to further extend and isolate the layers. After a few rounds of purging, the group blended eMica nanosheets.
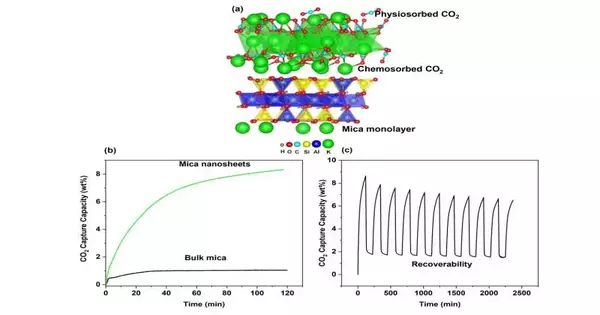
The lateral size and thickness of eMica nanosheet layers are more uniform than those of bulk mica. Moreover, eMica nanosheets display an arranged nuclear game plan, showing their excellent and insignificant deformities.
Partner Prof. Wu and his group next explored the capability of the nanosheets for CO2 capture applications. They discovered that eMica nanosheets had a CO2 adsorption capacity that was 87% greater than that of bulk mica. Even though other types of adsorption materials in the literature have shown a higher capacity, eMica nanosheets still outperformed other carbon-captured clay minerals.
The high specific surface area and porosity between the expanded layers of eMica nanosheets are to blame for the material’s superior capacity for CO2 adsorption. This two-dimensional material’s specific surface area had increased by more than fivefold, from 29.1 m2/g in bulk mica to 171.3 m2/g in nanosheets. The porosity of the nanosheets was additionally decisively higher, with the pore volume expanding seven-overlay, from 0.145 cc/g in mass mica to 1.022 cc/g in eMica nanosheets.
CO2 adsorption could likewise have been helped by stores of potassium carbonate (K2CO3) on the nanosheets, which are formed when potassium cations in mica react with water and CO2 in the air. Computer simulations showed that a mica monolayer deposited with K2CO3 outperformed both bulk mica and a mica monolayer in CO2 adsorption, corroborating the team’s hypothesis.
eMica nanosheets capture CO2 primarily through physical adsorption, which results in weaker electrostatic interactions with the surface. This contrasts with the weaker chemical absorption of CO2 on the nanosheet surface, which results in stronger ionic bonds. This overwhelming system of physisorption would take into account more straightforward CO2 desorption and the recovery of the eMica nanosheets.
The exploration group found the nanosheets had the option to keep up serious areas of strength with a limit when exposed to cyclic adsorption and desorption tests, showing the recoverability and dependability of the eMica nanosheets. Assoc Prof. Wu accepts this exploration will hold any importance with the power age area, ecological and administrative offices, and different scientists chasing after clever materials and innovations for CO2 capture. His research also contributes to SUTD’s plans for sustainability.
“One of the main areas of focus for the sustainability strategy of SUTD is CO2 capture, which is a crucial component of reducing emissions of greenhouse gases. “The university places an emphasis on sustainable operations as well as sustainable education and research, which is in line with our work on developing an effective synthesis method,” he said.
Assoc. Prof. Wu is moving forward with his plans to create a scalable method for exfoliating mica and investigate the potential uses of mica for water purification.
“Reducing carbon emissions and increasing energy efficiency could have significant repercussions for industry and society through the scalable fabrication of 2D materials using sustainable and cost-effective methods.” Generally speaking, we trust that this exploration will propel how we might interpret 2D materials and their possible applications and add to the improvement of manageable and inventive innovations,” he said.
More information: P. Vishakha T. Weerasinghe et al, Efficient Synthesis of 2D Mica Nanosheets by Solvothermal and Microwave-Assisted Techniques for CO2 Capture Applications, Materials (2023). DOI: 10.3390/ma16072921