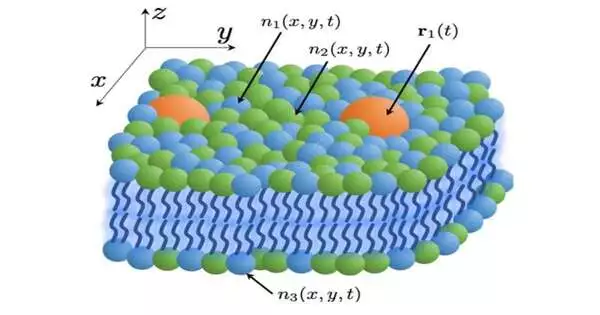
A theoretical model for more effective molecular-level simulations of cell membranes and their lipid-protein interactions has been developed by researchers at Lawrence Livermore National Laboratory as part of a multi-institutional effort to comprehend the behavior of cancer-causing membrane proteins.
The new model, which was developed as part of an ongoing collaboration between the Department of Energy (DOE) and the National Cancer Institute (NCI), aims to model cell membrane interactions with RAS, a protein whose mutations are linked to about 30% of human cancers. It solves a major issue in simulating RAS behavior, where conventional methods fail to reach the time and length scales needed to observe the biological processes of RAS-related cancers. The work is published in the most recent Physical Review Research issue.
Tomas Oppelstrup, a staff scientist at LLNL and co-author, stated, “We developed a continuum model in order to carry out simulations across greater lengths and timescales, in which one can trade spatial resolution for computational efficiency.” This model is unique in that it can describe any number of lipid species and is derived directly from the molecules’ microscopic properties, which can be calculated from direct molecular simulations.
“In order to carry out simulations across larger length and timelines, we devised a continuum model in which spatial precision can be traded for computational speed,”
LLNL staff scientist and co-author Tomas Oppelstrup.
The new model, which is based on Dynamic Density Functional Theory (DDFT), makes it possible to run simulations with resolutions that are close to those of the current gold standard for molecular dynamics (MD) models while still being able to access micron-level length scales and timescales on the order of seconds. These scales are, to put things in perspective, hundreds of times larger in space and many thousands of times longer in time than MD-accessible scales.
According to co-authors, the publication “rigorously and consistently” demonstrates that the DDFT framework is ideal for modeling multi-component cellular membranes as a continuum by incorporating the underlying molecular-level physics.
“Continuum models in this field have been previously restricted to one or two lipid types and phenomenological descriptions of the lipid interactions,” said first author Liam Stanton, a professor at San Jose State University who used to work as a staff scientist in the Center for Applied Scientific Computing at Lawrence Livermore National Laboratory.
“A method for maintaining molecular-scale accuracy at vastly larger length and timescales, inaccessible to MD simulations, has been provided by the DDFT formalism for an arbitrary number of lipid types.” At these scales, many new cycles of organic importance can be tested, and it will be invigorating to see what this new device will propose to malignant growth research and other natural networks.”
According to Fred Streitz, the project’s principal investigator and the LLNL Computing Directorate’s deputy associate director for strategic partnerships, the macroscopic model described in the paper is a “clever combination of continuum dynamics and particle dynamics and is constructed directly from finer-scale simulations.” Researchers were able to easily explore the possible space of protein arrangements and their lipid environments thanks to the model’s efficiency, which enables it to describe phenomena like lipid-driven protein aggregation.”
The framework was developed as part of the NCI/DOE Joint Design of Advanced Computing Solutions for Cancer (JDACS4C) Pilot 2 project, which aimed to improve comprehension of RAS-RAF-driven cancer initiation and growth by combining the cutting-edge experimental capabilities of the NCI to study RAS biology on membranes and advance the discovery of new cancer drugs with machine learning (ML) and high-performance computing expertise at DOE national laboratories.
In the pilot project, researchers from LLNL demonstrated a multiscale machine-learned modeling infrastructure (MuMMI) that could simulate the behavior of RAS proteins on a real cell membrane and how RAS-RAF proteins interact with membrane lipids and each other.
Through the use of next-generation experimental data, simulations, and predictive models, the objective was to improve comprehension of RAS biology. Simulations of RAS and its interactions with the cell membrane will, according to the researchers, improve our understanding of biology and provide new insights that will speed up the development of new cancer treatments that target RAS.
ADMIRRAL (AI-Driven Multiscale Investigation of the RAS/RAF Activation Lifecycle) is the follow-on project that expands on Pilot 2’s significant ability to model RAS biology to investigate signal-activation pathways over a much longer timescale.
Researchers claim that potential configurations along a path will be hypothesized and tested using machine learning, all without the need for human intervention. Streitz and Cancer Research Technology Program Director Dwight Nissley, both of the NCI’s Frederick National Laboratory for Cancer Research, co-direct ADMIRRAL at LLNL.
Adding more precise anisotropic protein interactions and membrane deformations to the recently published DDFT model is the goal of the researchers in the future. Tim Carpenter, Helgi Ingolfsson, Mike Surh, Felice Lightstone, and Jim Glosli, scientists at LLNL, were also co-authors.
More information: L. G. Stanton et al, Dynamic density functional theory of multicomponent cellular membranes, Physical Review Research (2023). DOI: 10.1103/PhysRevResearch.5.013080