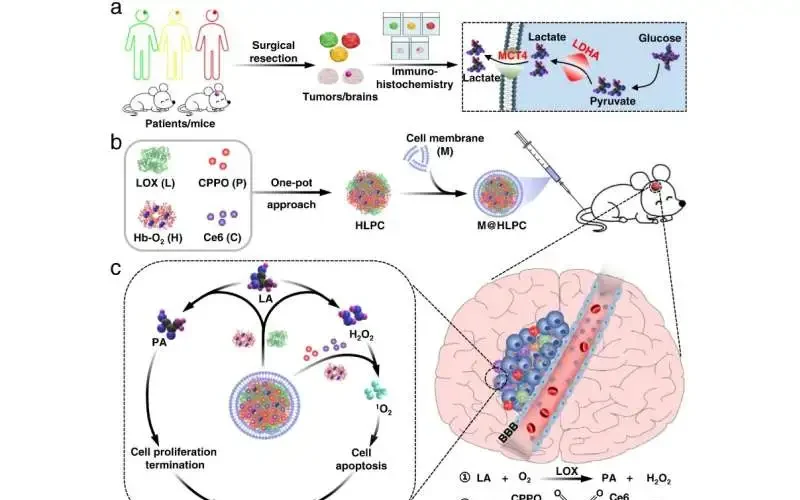
Glioblastoma multiforme (GBM) is a serious brain disease with an unfortunate prognosis and hardly any treatment choices. New and successful methodologies for GBM treatment are consequently critically required.
In light of the perception of raised lactate in resected GBM, scientists from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences and Shenzhen Second People’s Hospital have fostered a biomimetic plan involving designated conveyance specialists for lactate digestion based synergistic treatment against GBM.
The review was published in Nature Communications on July 21.
Focusing on lactate digestion is an appealing cancer restorative system. In any case, there are no reports that straightforwardly tackle lactate digestion for GBM medicines. One limit is the presence of the blood-mind hindrance, which forestalls most medication particles (counting those obstructing lactate digestion) from arriving at the cerebrum.
Besides, taking into account the intricacy and penetrating attributes of GBM, lactate metabolic monotherapy is probably not going to wipe out GBM cells actually. Hence, it is essential to foster synergistic techniques to upgrade the restorative productivity of lactate metabolic treatment.
In this review, the scientists gathered glioma tests from a huge number of patients and measured the lactate metabolic pointers LDHA and MCT4 and a delegate expansion marker Ki67.
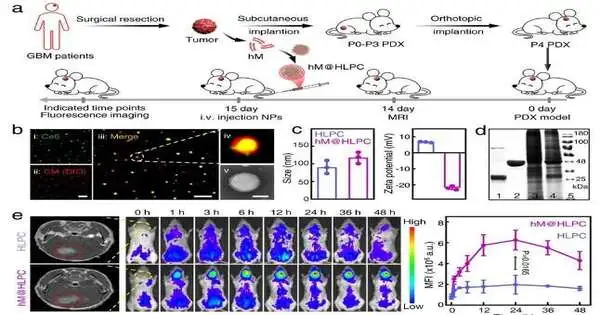
Fig. 2: Evaluation of the synergistic remedial impact of customized biomimetic definition in the PDX model.
“We noticed a positive connection between lactate metabolic markers and the degree of glioma multiplication,” said Prof. Li Weiping from Shenzhen Second People’s Hospital. In this way, a proficient digestion-based synergistic treatment was recommended that would straightforwardly tackle the raised lactate in GBM.
The scientists created self-getting nanoparticles (NPs) made out of hemoglobin (Hb), lactate oxidase (LOX), bis [2,4,5-trichloro-6-(pentyloxycarbonyl)phenyl] oxalate (CPPO), and chlorin e6 (Ce6) utilizing a one-pot approach. They accordingly typified these self-collected NPs with layer materials arranged from U251 glioma cells to produce the biomimetic M@HLPC framework. This plan idea had the option to accomplish designated conveyance for blend treatment.
“After intravenous infusion, the M@HLPC could cross the blood-brain obstruction by means of transcytosis obtained from integrin and vascular cell-attachment protein-interceded acknowledgment and afterward amassed in GBM through homotypic acknowledgment in view of cell-acknowledgment capability related proteins,” said Prof. Wei from IPE.
In cancers, LOX in the NPs changes lactate into pyruvic corrosive and hydrogen peroxide (H2O2). The pyruvic corrosive repressed disease cell development by hindering histone articulation and activating cell-cycle capture. The H2O2 acted as a nearby fuel to respond with the conveyed CPPO to deliver energy, which could then be utilized by the co-conveyed photosensitizer Ce6 for the generation of cytotoxic singlet oxygen to kill glioma cells.
Strong restorative adequacy was affirmed in both cell-line-determined xenograft and patient-determined xenograft (PDX) cancer models.
“Taking into account the wellbeing of the detailing and the powerful helpful impacts of the matched PDX model, our customized biomimetic plan can possibly mean clinical application,” said Prof. Mama Guanghui from IPE.
More information: Guihong Lu et al, Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma, Nature Communications (2022). DOI: 10.1038/s41467-022-31799-y