In a notable report presently distributed in the journal Nature Correspondences, the secrets of nitrogen’s strong stages have been settled, revealing insight into its mind-boggling conduct.
The exploration, driven by the College of Bayreuth as a team with researchers from the College of Edinburgh, U.K., and the College of Linköping, Sweden, gives uncommon bits of knowledge into the continuous sub-atomic to polymeric change of nitrogen and the development of undefined nitrogen. This makes it ready for progress in materials science and high-pressure physical science.
At the surrounding strain and temperature, nitrogen is gas and is tracked down as a N2 particle (N≡N) made out of an incredibly impressive triple bond. At the point when outrageous tensions are applied to sub-atomic vaporous nitrogen, it initially becomes fluid and afterward becomes strong at around 2.5 GPa (i.e., multiple times the climatic strain).
For over a really long period, researchers have dug into strong periods of sub-atomic nitrogen, as information on the chemico-actual components supporting changes in nitrogen is fundamental in testing and refining hypotheses of strong state sciences.
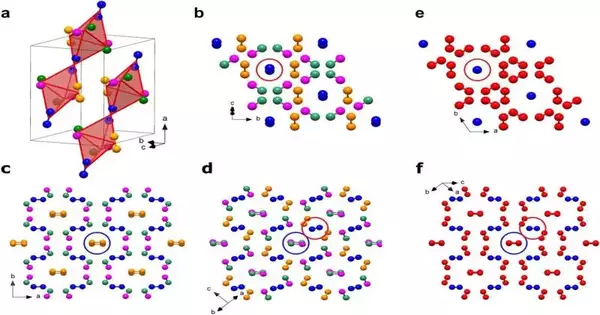
The Zeta-N2 period of nitrogen, existing somewhere in the range of 60 and 115 GPa, is a basic piece of the puzzle for understanding nitrogen’s sub-atomic to polymeric progress. In any case, notwithstanding countless examinations, its gem structure (i.e., the nitrogen particles’ course of action) was up until recently obscure and key to unraveling nitrogen’s odd way of behaving.
The exploration group, led by Dominique Laniel (College of Edinburgh) and Natalia Dubrovinskaia and Leonid Dubrovinsky (the College of Bayreuth), utilized a trial procedure recently created in Bayreuth to effectively decide the gem construction of Zeta-N2.
The analysts crushed atomic nitrogen in jewel blacksmith’s iron cells to outrageous tensions somewhere in the range of 60 and 85 GPa, like those predominant in the world’s mantle. By applying laser warming up to 2,000°C, they had the option to recrystallize great submicrometer-size grains of Zeta-N2. Their gem structure was settled and refined by synchrotron single-gem X-beam diffraction. With these exploratory discoveries close by, theoreticians at the College of Linköping (Sweden) acquired further experiences into nitrogen’s one-of-a-kind polymerization process.
The ramifications of this examination extend beyond nitrogen itself, offering a more profound comprehension of sub-atomic changes under outrageous circumstances. The discoveries make way for headways in strong state sciences, materials science, and high-pressure physical science. The scientists have worked on techniques for concentrating on the properties of practical materials utilized in hardware, microprocessors, semiconductors, solar-based cells, batteries, lighting, metals, or covers.
More information: Dominique Laniel et al, Structure determination of ζ-N2 from single-crystal X-ray diffraction and theoretical suggestions for the formation of amorphous nitrogen, Nature Communications (2023). DOI: 10.1038/s41467-023-41968-2