Charging and releasing a battery cell changes its cathode material into a “super” material.
Throughout the past 10 years, advances in innovative work have prompted more effective lithium-particle batteries. However, huge weaknesses remain. One test is the requirement for quicker charging, which can assist with speeding up the reception of electric vehicles.
An exploration group led by Boise State College and the College of California San Diego has adopted an unusual strategy to address this issue. Utilizing the assets of the U.S. Department of Energy’s (DOE) Argonne Public Lab, they made an elite battery cathode. The compound, niobium pentoxide, has a clever glasslike structure. It shows the guarantee for accelerating charging while at the same time giving a great stockpiling limit.
The group’s review was published in Nature Materials in May 2022.
During charging, lithium particles move from the positive terminal (cathode) to the negative anode (anode), usually made of graphite. At higher charging speeds, lithium metal will in general amass on the graphite’s surface. This impact, known as plating, will generally debase execution and can make batteries impede and burst into flames.
“The collaboration with Argonne experts was extremely beneficial in this work to uncover the unusual transition in niobium pentoxide. It also profited from its proximity to the APS, the Electrochemical Discovery Laboratory, and the CNM.”
Claire Xiong, the study’s lead investigator
Niobium pentoxide is considerably less helpless to plating, possibly making it more secure and more solid than graphite. Also, its iotas can organize in various stable setups that don’t need a lot of energy to reconfigure. This presents open doors for analysts to find new designs that could improve battery execution.
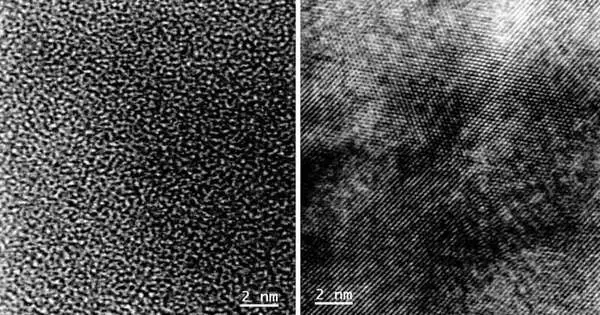
For this review, the scientists fabricated a coin cell with niobium pentoxide as the cathode material. (A coin cell, otherwise called a button cell, is a little round-molded battery gadget.) The niobium pentoxide had a nebulous design — as such, a confused plan of iotas. When the cell was charged and released various times, the confused design changed into an ordered, glasslike one. This particular design was never described in the logical writing.
Compared with the confusing plan, the glasslike structure empowered the simpler, quicker transport of lithium particles into the anode during charging. This tracking down focuses on the material’s commitment to quick charging, and different estimations propose that it can store a lot of charge.
Argonne gives a few integral devices.
Due to the intricate changes during the charge-release cycle, a few integral indicative devices were required for a complete understanding. That is where Argonne—and a couple of DOE Office of Science client offices at the lab—came in.
Yuzi Liu, a researcher in Argonne’s Center for Nanoscale Materials (CNM), utilized a method called transmission electron microscopy to check the primary change from nebulous to glasslike. This method sends high-energy electrons radiating through a material example. It makes for advanced pictures in view of the connection of the electrons with the example. The pictures show how iotas are organized.
“Since the electron bar is centered around a little region of the example, the method gives definite data about that specific region,” said Liu.
Hua Zhou, a physicist at Argonne’s High Level Photon Source (APS), affirmed the primary change with one more method known as synchrotron X-beam diffraction. This includes raising a ruckus around town with high-energy X-beam rays, which are dispersed by the electrons of the iotas in the material. A finder estimates this dispersion to portray the material’s design.
For example, X-beam diffraction is viable for giving data about generally primary changes across a whole material example. This can be useful in concentrating on battery anode materials on the grounds that their designs will generally shift starting with one region and moving onto the next.
“By raising a ruckus around the material with X-beam radiates at various points, I affirmed that it was consistently glasslike along the surface and on the inside,” said Zhou.
The exploration, likewise, drew upon other Argonne abilities for portraying materials. For Justin Connell, a materials researcher in Argonne’s Electrochemical Revelation Lab, utilized a device called X-beam photoelectron spectroscopy to assess the anode material. Connell’s shot X-beam radiates into the anode, shooting electrons from it with a specific energy.
“The method uncovered that niobium iotas gain various electrons as the cell is charged,” said Connell. “This proposes that the anode has a high stockpiling limit.”
Argonne physicist Sungsik Lee likewise assessed niobium’s benefit and loss of electrons. He utilized another method called X-beam ingestion spectroscopy. This involves raising a ruckus around town by material with extreme synchrotron X-beam bars and estimating the transmission and retention of the X-beams in the material.
“The method gave a general image of the condition of the electrons across the whole anode,” said Lee. “This affirmed that niobium acquires various electrons.”
Argonne is strange in that it has this multitude of examination abilities on its grounds. Claire Xiong, the review’s lead examiner, did her postdoctoral examination at Argonne’s CNM prior to joining the Boise State staff as a materials researcher. She was intimately acquainted with Argonne’s broad abilities and had recently teamed up with the Argonne researchers who added to the review.
“The offices and staff at Argonne are elite,” said Xiong. “This work to find the novel change in niobium pentoxide benefited hugely from the cooperation with Argonne researchers. It likewise profited from the admittance to the APS, Electrochemical Disclosure Lab, and CNM. “
It is truly challenging to make the elite exhibition glasslike niobium pentoxide with customary blend techniques, for example, those that subject materials to intensity and strain. The offbeat union methodology utilized effectively in this review—charging and releasing a battery cell—could be applied to make other creative battery materials. It might actually try to back up the creation of novel materials in different fields, like semiconductors and impetuses.
More information: Pete Barnes et al, Electrochemically induced amorphous-to-rock-salt phase transformation in niobium oxide electrode for Li-ion batteries, Nature Materials (2022). DOI: 10.1038/s41563-022-01242-0
Journal information: Nature Materials