Carbon dioxide (CO2) is the most plentiful ozone-harming substance causing environmental change and has existed on Earth well before people began delivering it into the climate at uncommon levels. Thusly, a portion of the planet’s earliest organic entities developed to outfit and utilize this gas, which is generally destructive to people and the planet.
One of those cycles, called the Wood-Ljungdahl pathway, just happens without a trace of oxygen and is believed to be the most effective carbon-obsession pathway in nature. Be that as it may, precisely the way in which the pathway continues, starting with one stage and then onto the next, has remained hazy.
Presently, researchers at the Stanford Synchrotron Radiation Lightsource (SSRL) at the Division of Energy’s SLAC Public Gas Pedal Lab, the College of Michigan, Northwestern College, and Carnegie Mellon College have found the previously obscure inward activities of the Wood-Ljungdahl pathway.
“Before this study, we knew that the Wood-Ljungdahl pathway starts with carbon dioxide in order to generate carbon for organisms to use. Then it transforms CO2 to carbon monoxide and a methyl group, which it then combines into a form of carbon that the organism can utilise.”
Macon Abernathy, a research associate at SSRL and co-author of the study.
Their discoveries, distributed in the Diary of the American Compound Society last month, not only revealed insight into quite possibly the most seasoned synthetic response on the planet, but may likewise prompt better carbon capture methods for environmental change relief endeavors.
“Before this review, we knew that for the Wood-Ljungdahl pathway to create carbon for organic entities to utilize, it begins with carbon dioxide,” said Macon Abernathy, an exploration partner at SSRL and co-creator of the review. “Then, at that point, it switches CO2 over completely to carbon monoxide and a methyl bunch and, through some sort of science enchantment, blends them into a type of carbon that can be utilized by the organic entity.”
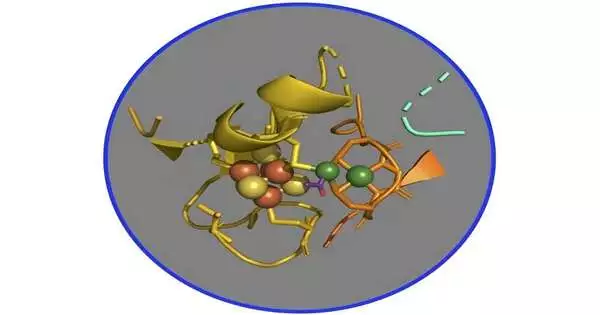
For a really long time, researchers hypothesized that the pathway works through a progression of nickel-based organometallic intermediates, which structure metal-carbon bonds. In particular, scientists have zeroed in on a complex of two nickel-iron-sulfur proteins called CO dehydrogenase and acetyl-CoA synthase (CODH/ACS), which are the essential chemicals that catalyze the change of carbon dioxide into energy and primary carbon for building cell walls and proteins.
Yet, affirming this speculation has shown precariousness as the need might arise to be cleansed in an environment lacking oxygen, similar to that of early Earth a long time ago when these proteins and this pathway arose. Moreover, the middle mixtures are often unsteady, and the response can immediately become dormant. Likewise, the presence of different iotas of nickel and iron in the CODH impedes the investigation of ACS, the objective of this review.
To evade these difficulties, the scientists fostered an additional dynamic, ACS-just form of the protein—no CODH—and utilized X-beams at SSRL to comprehend its metals and the way in which they work inside the chemical. The group applied X-beam spectroscopy, a strategy in which researchers concentrate on the obstruction of light that is consumed by, let out of, and afterward returned to the metals in a complex—here the ACS—to distinguish changing substance securities as the responses occur.
So, the researchers affirmed their well-established speculation.
“What we found is that there is an extremely multifaceted piece of organometallic science that is going on where a solitary nickel site in the chemical is doing all the tomfoolery stuff,” said Ritimukta Sarangi, senior researcher at SSRL and a comparing creator on the review.
That’s what the group discovered, albeit that the compound has a bunch of two nickels bound to four iotas each of iron and sulfur, and the response generally happens at one explicit nickel inside the bunch, said Steve Ragsdale, a teacher at the College of Michigan and a contributor to the review. “The carbons, for example, carbon monoxide, the methyl bunch, and the acetyl bunch, all tie to the nickel nearest to the iron and sulfur, and obviously they don’t tie to any of the different metals.”
The analysts additionally saw that the nickel-containing protein goes through significant changes in its construction at every last one of the moderate states, Ragsdale said. “That is something that is not actually part of our unique theory. We were simply pondering the fundamental science of nickel-based However, at that point, we saw this multitude of different changes that happened in the protein, which was somewhat amazing.”
Albeit the specialists had deep-seated thoughts regarding how the response functions, seeing it in real life was in any case noteworthy, said Abernathy.
“It’s such an exact adjusting of nature to show up at this exquisite framework that does this catalysis,” Sarangi said. “I simply love that and our capacity to utilize X-beam spectroscopy, which is a very useful asset for sorting out what’s happening in nature. SSRL’s Primary Sub-atomic Science Asset has a world-driving natural X-beam spectroscopy program that empowers the investigation of such complex organic cycles.”
Other than his appreciation for the normal excellence of the Wood-Ljungdahl pathway itself, Ragsdale said he trusts that exploration to more readily figure out these regular cycles and maybe improve those cycles could prompt strategies for relieving environmental change and creating carbon capture to make synthetic feedstocks and powers. “I feel that we need to figure out the fundamental organic chemistry behind the cycle first,” he said, “before we’ll have the option to gain ground in upgrading a portion of these pathways as they exist in nature.”
More information: Mehmet Can et al, Characterization of Methyl- and Acetyl-Ni Intermediates in Acetyl CoA Synthase Formed during Anaerobic CO2 and CO Fixation, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c01772