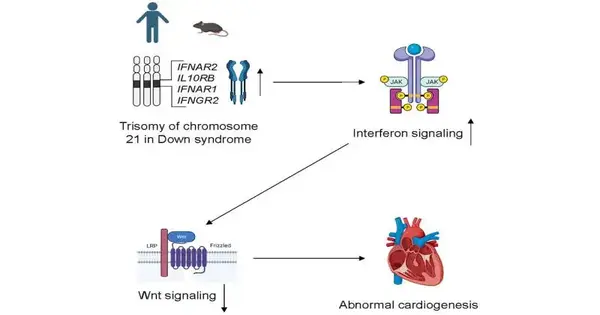
Congenital heart defects are extremely common in children born with Down syndrome, or trisomy 21, a genetic condition caused by an extra copy of chromosome 21. A congenital heart abnormality is present in nearly half of newborns with Down syndrome, and Down syndrome is recognized as the most common cause of congenital heart abnormalities. The mechanisms by which trisomy 21 prevents the proper formation of the heart during embryonic development remain unknown, despite numerous decades of research.
Researchers at the University of Colorado Anschutz Medical Campus recently discovered a molecular mechanism that contributes to Down syndrome’s defective heart development. A combination of experiments using human cells with and without trisomy 21 and a mouse model of Down syndrome were used by the research team, which was led by associate professor of medicine Dr. Kunhua Song, to illuminate the molecular basis of impaired heart formation.
The study has been published in iScience.
“We were surprised to see such high interferon activity during pluripotent stem cell differentiation into heart muscle cells with trisomy 21. We now understand that an abnormally large interferon response may be harmful to early heart development.”
Dr. Kunhua Song, associate professor of medicine,
The team simulated the initial stages of heart formation, or cardio genesis, in the laboratory with pluripotent stem cells obtained from participants in the Human Trisome Project, a large study of people with Down syndrome led by the Linda Crnic Institute for Down syndrome at the University of Colorado. They discovered that hyperactivity in the cellular response to viral infections, known as the interferon response, was linked to an impairment in cardio genesis caused by trisomy 21. Then, in the laboratory, they demonstrated through genetic and pharmacological methods that inhibiting the interferon response enhanced cardio genesis.
“We were surprised to observe strong interferon response activity during pluripotent stem cell differentiation into trisomy 21 heart muscle cells. Dr. Song elaborates, “We now appreciate that an abnormally high interferon response may be detrimental to early heart development.”
The researchers carried on their investigation into the specific mechanism by which interferon hyperactivity harmed cardiogenesis. As a result, they came to the conclusion that the interferon response inhibits a number of crucial molecular processes that are necessary for the development of the heart, such as the Wnt signaling pathway.
“Too much interferon activity leads to too little Wnt signaling, which in turn impairs heart muscle cell function,” says Dr. Congwu Chi, the paper’s lead author. By reducing interferon signaling or increasing Wnt signaling, this chain of events sheds light on possible treatments for Down syndrome’s abnormal heart formation.
The researchers administered a drug known as a JAK inhibitor to pregnant female mice in order to test this therapeutic approach in a Down syndrome mouse model. They also monitored the effects on heart development in embryos with a genetic mutation equivalent to trisomy 21. In fact, the JAK inhibitor improved the cardiogenesis of pregnant mice significantly.
“Because they suggest a potential pharmacological strategy for prenatal treatment of one of the most harmful effects of trisomy 21, these results are very important. Congenital heart defects and Down syndrome present a number of challenges, including the need for immediate heart surgery and long-term effects on physiology. However, “to define the safety and efficacy of using JAK inhibitors during pregnancy,” says Dr. Joaquin Espinosa, executive director of the Linda Crnic Institute for Down syndrome and co-author of the paper, “will require a lot of additional research.”
Currently, Dr. Espinosa and his team are leading clinical trials to investigate the benefits of JAK inhibitors for older Down syndrome children and adults.
These findings add to the growing body of evidence that interferon hyperactivity has negative effects on Down syndrome, even in the early stages of embryonic development. The findings also lend credence to the hypothesis that immune dysregulation, which persists throughout a person’s life, is the root cause of many of Down syndrome’s symptoms and that reestablishing this equilibrium may have therapeutic potential.
According to Dr. Espinosa, “This body of work is proof of the rapid scientific advances that can occur with the right investment of resources and the willingness of individuals with Down syndrome to participate in research.”
More information: Congwu Chi et al, Interferon hyperactivity impairs cardiogenesis in Down syndrome via downregulation of canonical Wnt signaling, iScience (2023). DOI: 10.1016/j.isci.2023.107012