Writing in Nature Correspondences, a group driven by Dr. Marcelo Lozada-Hidalgo based at the Public Graphene Foundation (NGI), utilized graphene as a cathode to gauge both the electrical power applied to water particles and the rate at which they break because of such power. The analysts found that water breaks dramatically quicker because of more grounded electrical power.
The analysts believe that this key understanding of interfacial water could be utilized to better configure impetuses to create hydrogen fuel from water. This is a significant piece of the U.K.’s system towards accomplishing a net zero economy. Dr. Marcelo Lozada-Hidalgo said, “We trust that the experiences from this work will be useful to different networks, including physical science, catalysis, and interfacial science, and that it can assist with planning better impetuses for green hydrogen creation.”
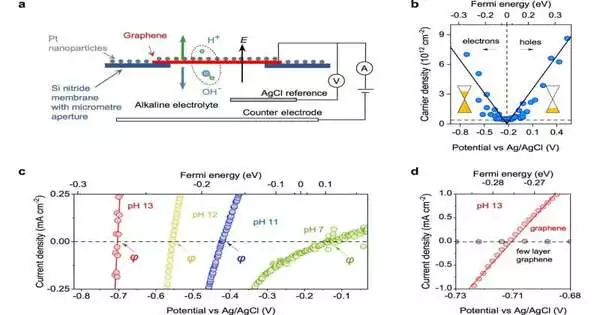
A water particle is comprised of a proton and a hydroxide particle. Separating it includes pulling these two constituent particles separated with an electrical charge. On a basic level, the more grounded one pulls the water particle apart, the quicker it ought to break. This significant point has not been shown quantitatively in tests.
Electrical powers are known to break water particles, yet more grounded powers don’t necessarily prompt quicker water separation, which has baffled researchers for quite a while. A vital contrast with graphene cathodes is that they are porous just to protons. The scientists found that this enables isolating the subsequent proton from the hydroxide particle across graphene, which is a one-iota thick boundary that forestalls their recombination. This charge division is fundamental to noticing the electric field speed increase of water separation. One more key benefit of graphene is that it permits assessing the electric field at the graphene-water interface tentatively, which considers a quantitative portrayal of the field impact.
“We anticipate that the insights gained from this work will be useful to a variety of communities, including physics, catalysis, and interfacial research, and that they will aid in the development of improved catalysts for green hydrogen production.”
Dr. Marcelo Lozada-Hidalgo
The results can be explained using the old-fashioned Onsager hypothesis, which has remained tentatively unconfirmed in the significant case of water.Junhao Cai, a Ph.D. understudy and co-first creator of the work, said, “We were amazed to find how well the Onsager hypothesis fitted our information. This hypothesis gives experiences into interfacial water, including a free gauge of its dielectric steady, which remains inadequately comprehended. “
The creators are amped up for the potential outcomes presented by their trial arrangement. Eoin Griffin, Ph.D. understudy and co-first creator of the work, said, “Graphene cathodes join three properties that, supposedly, are never tracked down together in a solitary framework: just protons pervade through the gem, it is one-iota thick, and it can have areas of strength for support powers. This mix permits us to basically pull apart the main layer of water atoms on the graphene surface.
More information: J. Cai et al, Wien effect in interfacial water dissociation through proton-permeable graphene electrodes, Nature Communications (2022). DOI: 10.1038/s41467-022-33451-1
Journal information: Nature Communications