Skoltech scientists, with the assistance of their partners from Moscow State University, revealed the charge stockpiling systems of NiBTA—an as of late discovered material that can empower quick charging batteries. Their report is distributed in Chemical Science.
For example, quick charging batteries are required for some applications, for example, electric vehicles that experience the ill effects of the “range tension” issue. Nonetheless, current battery anode materials are either inadmissible for solid quick charging or bear the cost of just moderate energy thickness. It spurs the researchers to look for new materials that have bigger limits and can securely work under quick charging conditions.
“This aided in obtaining reliable results because each method provides only a portion of the image. We employed operando X-ray diffraction and operando Raman spectroscopy, among other techniques, to trace the structural changes inside the batteries in great detail. We were the first to use such a stringent method for this family of chemicals. This research illuminates the redox chemistry of coordination polymers, which can be valuable in a variety of applications.”
Skoltech Professor Roman Kapaev
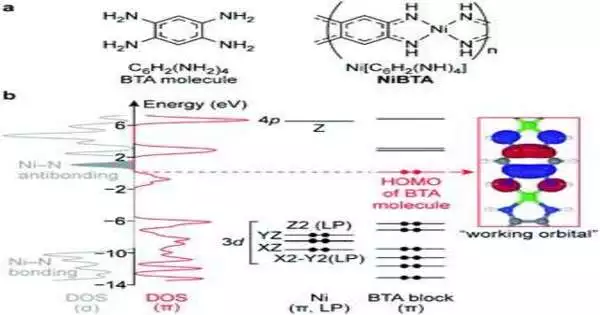
Recently, scientists proposed NiBTA, a nickel-based coordination polymer derived from benzenetetramine, as another promising anode material. Nonetheless, it remains hazy how NiBTA is charged and released in batteries. Different exploration groups proposed various systems, generally on the grounds that the information was too vague to give solid ends. In the new work, Skoltech researchers utilized a mix of cutting-edge techniques to acquire experience of NiBTA conduct in lithium-, sodium-, and potassium-based batteries.
“The excellence of this work is in assembling different methods, both trial and hypothetical,” the main creator of the review, Skoltech Professor Roman Kapaev, said. “This assisted with obtaining reliable outcomes on the grounds that every strategy gives just a piece of the picture.” In addition to other things, we utilized operando X-beam diffraction and operando Raman spectroscopy, which permitted us to follow the primary changes inside the batteries exhaustively. We were quick to apply such a thorough methodology to this class of mixtures. This study reveals insight into the redox science of coordination polymers, which can be helpful for some applications. “
More information: Roman R. Kapaev et al, Charge storage mechanisms of a π–d conjugated polymer for advanced alkali-ion battery anodes, Chemical Science (2022). DOI: 10.1039/D2SC03127B