Parkinson’s disease, a progressive and crippling neurodegenerative disorder that affects millions of people worldwide, may be closely linked to two proteins.
Researchers are hustling to find new pieces of information about the condition, whose pervasiveness is expanding due to the inflexible maturing of the worldwide populace. The majority of cases occur after the age of 60, but an estimated 5 to 10 percent are early-onset forms that begin before the age of 50. These cases frequently, but not always, involve a genetic mutation that is passed down through generations.
Parkinson’s is set apart by degeneration of the mind’s basal ganglia and a lack of the essential synapse dopamine. The disorder is characterized by tremor, muscular rigidity, and a slow, imprecise gait. Patients are further impacted by a complex and disparate range of non-motor symptoms that can significantly impact their quality of life, despite the severity of those symptoms.
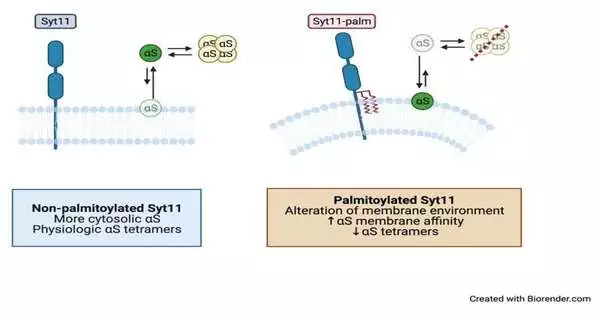
“We studied whether the two proteins were linked. We discovered that Syt11 was palmitoylated in mouse and human brain tissue, as well as in cultured cortical neurons, and that this alteration to Syt11 impaired -synuclein homeostasis in neurons.”
Dr. Gary P.H. Ho , lead author of the study reported in the journal Science Signaling.
According to the Parkinson’s Foundation, a U.S. nonprofit that serves as an information resource for patients and their families, depression, anxiety, apathy, hallucinations, constipation, sleep disorders, loss of sense of smell, and a variety of other cognitive impairments can make it more difficult for patients to cope with the condition.
A new study has revealed a connection between two proteins that accelerate the disease’s progression and has focused on crucial molecular mechanisms that are at the heart of the condition.
These proteins have been identified as synaptotagmin-11 (Syt11) and alpha-synuclein, more commonly referred to as synuclein, by Dr. Gary P.H. Ho and colleagues from the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital in Boston. Ho and colleagues discovered that this rebellious pairing probably plays a role in the disease’s characteristic cellular pathology.
The new research clarifies the biochemical link between Syt11 and alpha-synuclein, a key brain driver of Parkinson’s disease, and may explain why Syt11 was previously linked to the disease, despite its role remaining elusive up until this point. The scientific understanding of Syt11 has been enhanced by the new, in-depth examination of its activity in conjunction with synuclein.
The study’s lead author, Ho, writes, “We investigated whether the two proteins were connected.” The study was published in the journal Science Signaling. We discovered that palmitoylation of Syt11 disrupted synuclein homeostasis in neurons in cultured cortical neurons, as well as in human and mouse brain tissue.”
The covalent attachment of fatty acids, like palmitic acid, to cysteine (and less frequently to serine and threonine) is known as palmitoylation. Ho and colleagues discovered in the study that palmitoylation affected two cysteine residues on Syt11, causing the protein to move to areas of the intracellular membrane in neurons where it could not be broken down. The palmitoylation of Syt11 also increased the amount of the disease-associated, aggregation-prone form of -synuclein, as demonstrated by additional experiments.
Ho continued, “Palmitoylation of Syt11 increased its abundance in neurons and enhanced the binding of synuclein to intracellular membranes.” The abundance of the aggregation-prone monomeric form of -synuclein increased, while the abundance of the physiological tetrameric form decreased.”
Ho and his colleagues looked at brain tissue from both human donors and an animal model of Parkinson’s disease for the study. According to data published in Science Signaling, the two harmful proteins had an effect “in primary neurons from mice and in neurons derived from cells from healthy donors and a patient with familial Parkinson’s disease.”
Despite the fact that the research sheds new light on the molecular mechanisms underlying Parkinson’s disease, the disease’s cause remains elusive, and there is no cure. For a select few individuals whose diagnosis is explained by a genetic mutation, the neurodegenerative condition may have familial roots. Although scientists suggest that exposure to herbicides or pesticides may play a role, the majority of cases have no clear cause.
In recent years, a flurry of studies has made progress in understanding the disorder’s fundamental mechanisms and genetic risk factors. For instance, the gene that encodes Syt11 has been linked to an increased risk of Parkinson’s disease in genome-wide association studies.
The Boston team, all of whom work together in Dr. Dennis Selkoe’s lab, has now demonstrated how the two proteins are connected, taking the connection between Syt11 and alpha-synuclein to a tantalizing new level.
The Parkinson’s Foundation, with headquarters in Miami and New York City, states that the disease affects more than 10 million people worldwide. Levodopa, which treats the disease’s motor symptoms, is the disorder’s primary medication. The drug is processed by neurons to replace depleted dopamine. Carbidopa and other medications like it support the brain as a chronic disease progresses.
In contrast, Ho and colleagues assert that their research is expanding our understanding of Parkinson’s disease. This work starts to bring robotic lucidity to the job of Syt11 in the sickness and gives additional proof that palmitoylation impacts numerous pathways in the science of [-synuclein],” Ho finished up.
More information: Gary P. H. Ho et al, Palmitoylation of the Parkinson’s disease–associated protein synaptotagmin-11 links its turnover to α-synuclein homeostasis, Science Signaling (2023). DOI: 10.1126/scisignal.add7220