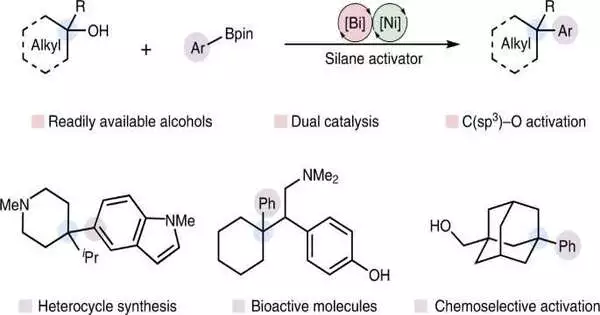
A group of researchers from the College of Ottawa has fostered a creative procedure to fabricate complex synthetic designs from effectively open substrates, making it one of the easiest and most commonsense strategies for changing alcohols into their arylated reciprocals.
This creative strategy for playing out the response, specifically the deoxygenative Suzuki-Miyaura arylation of aliphatic alcohols, utilizes two unmistakable metal impetuses. It is anticipated that their reaction will have a significant impact on the creation of new molecules and that it operates under mild reaction conditions with minimal waste products. As a result, it will help the pharmaceutical, agrochemical, and other related industries advance.
The review has been distributed in Nature Amalgamation.
Under the direction of Professor Stephen G. Newman, an associate professor of chemistry and biomolecular sciences at the Faculty of Science who holds a Tier 2 Canada Research Chair in Sustainable Catalysis, this study was carried out in the Newman Lab at the University of Ottawa. Adam Cook, a fifth-year Ph.D. candidate, was the research’s lead author, and Piers St. Onge, a third-year Ph.D. candidate, was the research’s second author.
“The chemical reaction that we have developed, making extensive use of the high-throughput labs in uOttawa’s Centre for Catalysis Research and Innovation, eliminates prior restrictions in Suzuki-Miyaura arylations by providing a surprisingly simple method to achieve the direct derivatization of a broad range of easily accessible alcohols.”
The lead author of the research was Adam Cook, a fifth-year Ph.D. candidate
Cook explains, “The chemical reaction that we have developed, making extensive use of the high-throughput labs in the uOttawa’s Centre for Catalysis Research and Innovation, eliminates previous limitations in Suzuki-Miyaura arylations by offering a surprisingly simple method to achieve the direct derivatization of a broad range of alcohols that are easily accessible.” This method can be used to derivatize a wide variety of alcohols.
“This method generates water as a waste product rather than metal-halide salts by using these molecules as starting materials instead of more established organohalides.” The current methods for Suzuki-Miyaura arylation, one of the most widely used chemical reactions in the world, require multiple synthetic steps to obtain the necessary starting materials. This reaction not only provides an effective method for generating complex, medicinally relevant structures from accessible materials, but it also contributes to fundamental developments in how chemical feedstocks may be directly transformed into important materials using catalysis.”
“We eliminate the need for these wasteful and time-consuming synthetic steps, thereby streamlining the process of converting naturally abundant substances into value-added products,” by developing a method that permits the direct use of naturally abundant alcohols in these transformations. Additionally, “through cross-coupling reactions via an SN1-type pathway, we were able to pursue a unique mechanistic hypothesis en route towards this goal,” Cook states.
This is an area of synthetic chemistry that hasn’t been studied much before, and the team of researchers hopes that their work will inspire other scientists. “High-throughput experimentation can assist you in reaching conclusions in a rapid and comprehensive manner, regardless of how “out there” your hypothesis may be,” Cook states.
Scientists can now produce a wide range of arylated alcohols with greater precision and efficiency than ever before by utilizing the power of this new method.
More information: Adam Cook et al, Deoxygenative Suzuki–Miyaura arylation of tertiary alcohols through silyl ethers, Nature Synthesis (2023). DOI: 10.1038/s44160-023-00275-w