Over 40 years into the HIV pandemic, researchers are as yet turning up signs uncovering how the infection seizes its host’s phone cycles to help its own replication — and advance the drawn out endurance of the infection.
Another review, driven by scientists at the College of Pittsburgh, has recognized how a HIV protein called Nef enacts compound action in cells vital for the infection to repeat.
While it may appear that researchers have unwound the numerous secrets fundamental to the HIV disease process, the new study is a declaration to the complexities of an infection that has killed over 40 million people since the mid 1980s but has yet to be cured.
“Infections guarantee fruitful replication, to some extent, by undermining host flagging pathways,” writes Dr. Manish Aryal of the Branch of Microbial Science and Sub-atomic Hereditary Quality at the College of Pittburgh’s Institute of Medication. “The Nef protein, which is produced by the infections HIV-1 and SIV [simian immunodeficiency virus], promotes viral replication by inducing constitutive activation of host cell tyrosine kinases.”
HIV-1 is the microbe that makes help and proceeds to fuel the continuous overall pandemic. SIV is a connected, irresistible specialist that taints non-human primates.
“Viruses assure successful replication in part by subverting host signaling pathways, such as the Nef protein generated by the viruses HIV-1 and SIV (simian immunodeficiency virus), which drives efficient viral replication in part by causing constitutive activation of host cell tyrosine kinases.”
Dr. Manish Aryal of the Department of Microbiology and Molecular Genetics at the University of Pittburgh’s School of Medicine.
Meanwhile, tyrosine kinases are proteins that are essential to cell function, particularly as flagging atoms involved in the complex informing that occurs throughout cells.There are whole groups of tyrosine kinases, one of which is the Sleuth family. These kinases are engaged with intracellular flagging systems of cytokine receptors, G-protein-coupled receptors, lymphocyte surface antigens, and integrin atoms. They are also key players in the guidelines for safe cell capabilities.
The Src family is one more group of tyrosine kinases. Individuals are non-receptor tyrosine kinases that are primarily cell film related, acting as key flagging delegates in cycles such as cell separation, expansion, movement, digestion, and apoptosis.
Generally, tyrosine kinases can move a phosphate bunch from the energy particle adenosine triphosphate—ATP—to the tyrosine buildups of explicit proteins inside cells. They are, moreover, significant, paying little mind to which family since they go about as “on” and “off” switches in a huge number of cell capabilities. Due to their universality and significance to cell capability, HIV has figured out how to avoid tyrosine kinases to its own conceptual benefit.
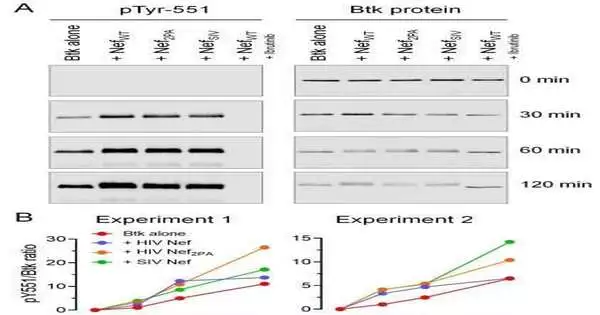
Writing in the diary Science Flagging, the Pittsburgh group, teaming up with analysts from the College of Iowa, revealed how HIV’s Nef protein enacts the chemical Btk [Bruton tyrosine kinase], which is basic for HIV replication. Nef advances primary changes in Btk, which really puts the protein under HIV’s influence. It achieves this errand by settling an intermolecular complex known as the SH3-SH2 area. After settling this area, the Btk protein is influenced quite a bit by and performs errands that benefit the infection, not the host’s cells.
The system engaged with the HIV-Btk connection is particular to how Btk is enacted in solid cells. HIV’s Nef protein likewise has a different playbook to enact compounds of the Src family. Yet, the take home message here is this: The HIV-Btk connection is a formerly unnoticed method of Sleuth family chemical guidelines and shows that HIV’s Nef protein can prompt tyrosine kinase enactment through free systems.
“HIV-1’s Nef protein advanced particular systems to enact Src and Sleuth family tyrosine kinases to improve viral replication,” Aryal noted.
Although the discoveries break new logical ground, they highlight a misleading basic point: As a rule, it has required a great many long periods of development for infections to consummate their abilities to surpass the sub-atomic systems of cells. Scientists like Aryal and others are working hard to unravel the plethora of messy tricks infections use to support themselves and thrive as microbes.The Pittsburgh-driven research adds to a developing list of HIV information that has expanded throughout recent years—and is still developing.
HIV, or human immunodeficiency infection, is a retrovirus whose hereditary material is made up of RNA. What’s more, since characterized as a retrovirus, HIV is furnished with a protein called switch transcriptase, or RNA-subordinate DNA polymerase. The protein enables the infection to change its RNA into DNA by blending a two-fold helix of DNA through switch transcriptase. The shrewd infection, practically like sorcery, makes its hereditary material like that of its host: twofold abandoned DNA.
This uncommon element permits the infection to make a huge number of duplicates of itself in host cells. As striking as that accomplishment might appear, it isn’t the main sub-atomic trait of HIV that has made it a worldwide irresistible danger. The infection can hang out in an idle period that can keep going for quite a long time. So its capacity to control tyrosine kinase, as Aryal and partners have found, ought to shock no one.
Aryal and his group deduce in their review that the Nef protein created by HIV advances viral replication and illness movement through a few systems, suggesting that hindering this protein might be a powerful procedure to control the sickness in tainted people.
More information: Manish Aryal et al, The HIV-1 protein Nef activates the Tec family kinase Btk by stabilizing an intermolecular SH3-SH2 domain interaction, Science Signaling (2022). DOI: 10.1126/scisignal.abn8359
Journal information: Science Signaling