In science, a particle is supposed to be chiral in the event that it can’t be superposed onto its perfect representation by any blend of revolutions, interpretations, or conformational changes. A chiral particle or particle exists in two structures, called enantiomers, that are identical representations of one another; they are frequently recognized as either ‘right-gave’ or ‘left-gave’ by their outright setup. Except when they interact with polarized light and react with other chiral compounds, enantiomers have similar chemical and physical properties.
Lately, isotopically chiral builds, in view of the segregation of isotopes of a component, have produced extensive interest in primary and manufactured natural science, restorative science, and principal response systems because of their clever properties and likely applications. Various optically dynamic isotopic particles with topsy-turvy carbon have been orchestrated in light of hydrogen/deuterium (H/D) segregation.
Notwithstanding, the blend, identification, and portrayal of isotopic atropisomers—sstereoisomers coming about because of ruined pivots about single bonds, wherein the steric strain obstruction to turn is sufficiently high to take into consideration the seclusion of the conformers—iis especially difficult. Only a small number of such molecules with H/D discrimination have been identified to date.
The asymmetric synthesis of isotopic atropisomers based on the discrimination of carbon isotopes has recently been successfully demonstrated by a group of researchers led by Professor Osamu Kitagawa of the Department of Applied Chemistry at the Faculty of Engineering at the Shibaura Institute of Technology in Japan. The results of their research were published online in The Journal of Organic Chemistry. It was co-created by Ryunosuke Senda and Yuka Watanabe, graduate understudies at the Division of Applied Science, Shibaura Establishment of Innovation.
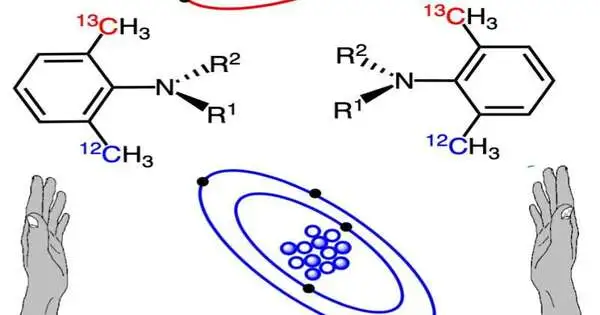
The team successfully prepared both enantiomers of 2-ethyl quinazolin-4-one with isotopic atropisomerism (N-C axial chirality) based on ortho-12CH3/13CH3 discrimination, drawing on previous work on the synthesis of CH3/CD3-atropisomeric quinazolin-4-one derivatives.
They accomplished this accomplishment through an unbalanced blend strategy, including Suzuki-Miyaura cross-coupling. “The orchestrated isotopic atropisomers in view of 12C/13C separation were cryptochiral compounds having no optical turn,” calls attention to Prof. Kitagawa.
The researchers then synthesized diastereomeric 3-aryl quinazolin-4-one derivatives with an asymmetric carbon atom and isotopic atropisomerism because they were unable to confirm that the aforementioned molecules had isotopic atropisomerism. Using 1H and 13C nuclear magnetic resonance, the diastereomers could be clearly distinguished and the isotopic atropisomerism could be confirmed.
They also had high rotational stability and stereochemical purity for diastereomers and enantiomers. Diastereomeric 3-aryl quinazolin-4-ones are, as a result, an excellent scaffold for confirming various isotopic atropisomers.
The novel findings of this study will undoubtedly improve our fundamental comprehension of isotopic atropisomers, which will have positive effects on organic and medicinal chemistry. According to Prof. Kitagawa, “This work reports the first isotopic atropisomers based on 12C/13C discrimination, which could potentially pique academic interest in researchers to conduct similar studies in fundamental organic chemistry.”
More information: Ryunosuke Senda et al, Synthesis of Isotopic Atropisomers Based on 12C/13C Discrimination, The Journal of Organic Chemistry (2023). DOI: 10.1021/acs.joc.3c01004