Industry has long depended upon energy-serious cycles, like refining and crystallization, to isolate atoms that eventually act as fixings in medication, synthetic substances, and different items.
In many years, there has been a push to override these cycles with films, which are possibly a cheaper and more eco-accommodating option. Tragically, most layers are produced using polymers that debase during use, making them unreasonable.
To take care of this issue, a College at Bison Drive research group has made a new, sturdier layer that can endure unforgiving conditions—high temperatures, high tension, and complex solvents—related to modern partition processes.
“The processes of separating molecules, whether for water desalination, medicine production, or fertilizer production, require enormous amounts of energy. What we’ve developed is a method for easily fabricating defect-free, strong membranes with rigid nanopores that can be precisely controlled to allow different-sized molecules to pass through,”
Miao Yu, Ph.D., SUNY Empire Innovation Professor in the Department of Chemical and Biological Engineering in the University.
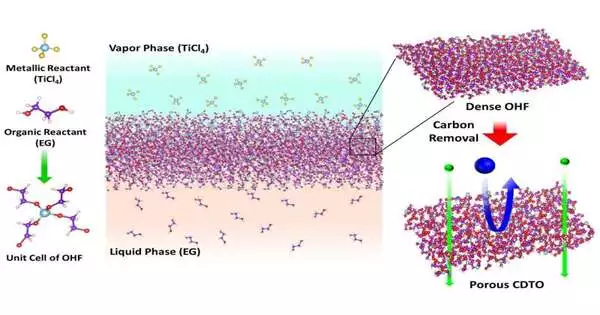
Produced using an inorganic material called carbon-doped metal oxide, it is portrayed in a review distributed on Sept. 7 in Science.
“The cycles of isolating particles—whether for water desalination, the creation of medication, or composts—utilize an extraordinary measure of energy,” says the review’s comparing writer, Miao Yu, Ph.D., SUNY Domain Advancement Teacher in the Branch of Substance and Natural Designing in the College at Bison School of Designing and Applied Sciences.
“What we have created is a method to effortlessly manufacture deformity-free, solid films that have unbending nanopores that can be unequivocally controlled to permit different-sized particles to go through,” adds Yu, a center employee at the UB Reestablish Foundation.
The concentrate’s most memorable creators are Bratin Sengupta, a Ph.D. understudy in Yu’s lab, and Qiaobei Dong, Ph.D., who concentrated under Yu and presently works at GTI Energy.
Enlivened by semiconductors
To make the film, the exploration group took inspiration from two normal, yet irrelevant, fabricating procedures.
The first is the atomic layer affidavit, which includes layering slender movies of materials and is most frequently connected with semiconductor creation. The subsequent procedure is interfacial polymerization, which is a strategy for joining synthetics that is usually used to make power modules, compound sensors, and other hardware.
“These strategies are not new,” says Sengupta, “but how we apply them is, and that is the way to making our new nanoporous films.”
In tests, scientists blended two minimal-cost reactants—fluid ethylene glycol and vaporous titanium tetrachloride—on an aluminum-based catalyst. In practically no time, the response made a meager film.
To make the nanopores, they applied intensity to the film. The intensity consumes carbon, making minuscule, infinitesimal openings for particles to go through. The size of the nanopores can be somewhere in the range of 0.6 to 1.2 nanometers in breadth still up in the air by the calcination gas climate, as well as the sum and span of intensity.
The technique permits scientists to stay away from an irritating issue—little openings converging into bigger ones, hence making them more permeable than expected—when making polymer-based films.
Potential to lessen carbon impression
The new film can endure temperatures up to 284°F (140°C) and pressures up to 30 psi when exposed to natural solvents. These characteristics are key since they permit the layer to isolate particles at high temperatures (for most polymer films to work, the temperature of the solvents should be brought down, which is exorbitant from an energy standpoint).
“Starting from this point of view, our layer has the potential to lessen the carbon impression of numerous modern cycles,” Yu says.
To exhibit the film’s viability, the group showed it could isolate boscalid, a fungicide used to safeguard crops, from its impetus and beginning reagent. The whole interaction happened at 194°F.
The group is arranging extra trials to demonstrate the layer is equipped to be increased for business items. Moreover, Yu plans to begin an organization to further the innovation’s business feasibility.
More information: Bratin Sengupta et al, Carbon-doped metal oxide interfacial nanofilms for ultrafast and precise separation of molecules, Science (2023). DOI: 10.1126/science.adh2404