When Eadweard Muybridge created the images that would later become motion pictures, his electrifying photographs of a galloping horse lit up the world. A recent upgrade to one of the most potent hard X-ray light sources in the world could make it easier for scientists to make molecular movies. These may open the door to new pharmaceuticals and treatments by revealing the secrets of various chemicals.
When attempting to reconstruct the molecular structure of proteins, researchers typically employ various forms of the method known as crystallography. A new technique known as serial crystallography, which was previously developed at X-ray free-electron laser facilities, has now been utilized and expanded by researchers at the Argonne National Laboratory of the United States Department of Energy (DOE). According to the review, a paper appeared to have nature correspondences.
Scientists are able to observe changes in the shape of bound molecules and proteins in real time during chemical reactions by combining serial crystallography with observations over short time scales (from a tenth to a hundredth of a second). The method has a distinct advantage over previous forms of crystallography because individual crystals can be smaller and only require a single, brief exposure to X-ray light.
“Serial crystallography is still in its infancy and has generally been the domain of specialist facilities with free-electron lasers. With the APS Upgrade, we will be able to analyze all kinds of processes as they occur, particularly for biological systems.”
Andrzej Joachimiak, who is also the director of the Structural Biology Center (SBC) at the APS.
X-ray beams that are up to 500 times brighter than those that are currently produced at Argonne’s Advanced Photon Source (APS), a DOE Office of Science user facility, will be produced by the upcoming upgrade. According to Argonne distinguished fellow Andrzej Joachimiak, who is also the director of the Structural Biology Center (SBC) at the APS and a professor at the University of Chicago, this will make serial crystallography available to a wider audience at the APS.
Joachimiak stated, “Serial crystallography is really in its infancy and has largely been the responsibility of specialized facilities with free-electron lasers.” We will be able to study all kinds of reactions in real time with the APS Upgrade, especially for biological systems.”
TR-SSX captured images of S. maltophilia moxalactam cleavage by L1 (20–4000 ms). Protein is represented by yellow, zinc ions by magenta, water molecules by red, and moxalactam by a stick, with carbon atoms shown in green prior to -lactam cleavage, orange immediately after cleavage (150 milliseconds), and blue in the final product during conformational adjustments. The video depicts changes in conformation of the moxalactam model within the L1 active site.
In a recent experiment with researchers at the University of Chicago, Joachimiak used serial crystallography to study the reaction of an antibiotic drug and an enzyme isolated from a drug-resistant pathogen. The outcome may help researchers gain a better understanding of the molecular mechanisms by which some bacteria acquire antibiotic resistance.
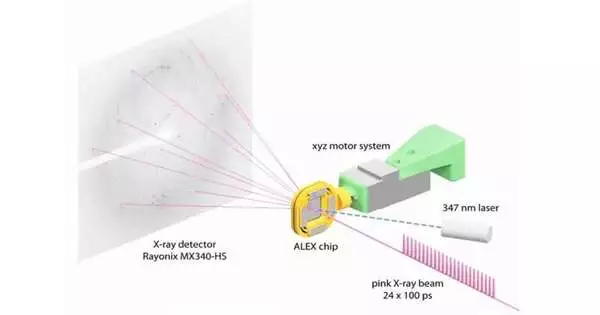
The BioCARS beamline at 14-ID, which is managed by the University of Chicago, and SBC at beamline 19-ID were utilized by the research team. Vukica Srajer, a scientist at the BioCARS research beamline who is also a co-author of the paper, says that the beamline is one of the few in the world that can do serial crystallography in a very short amount of time. BioCARS was used for time-resolved serial crystallography by the researchers.
Joachimiak and his colleagues were able to quickly and precisely reconstruct a number of protein structures by scanning an X-ray image of a plastic chip that contained a suspension of thousands of individual protein microcrystals. A “pump-probe” method in which ultraviolet light was shined on the sample to start a reaction was used by the researchers. The result was then observed at various times using the X-ray beam.
“Past efforts to do this sort of crystallography would annihilate the gems before we could get the total information,” Joachimiak said. “We can get more than 40,000 images from a single chip because we are only shining X-rays on each crystal for a very short time. This emphatically accelerates our crystallography endeavors and empowers us to see into protein instruments throughout a few time scales that we’d until recently never had the option to determine.”
According to Joachimiak, the advantage of performing serial crystallography is that it enables researchers to observe changes in the structure of the protein as they occur. It also enables researchers to examine how an enzyme’s active site interacts with a different molecule, or substrate, in the case of an enzyme.
Joachimiak and his colleagues examined a protein-enzyme complex known as a beta-lactamase, which confers antibiotic resistance on some pathogens. The researchers were able to observe a buildup of zinc atoms using serial crystallography, which was what caused the enzyme to break through the antibiotic molecule.
Joachimiak stated, “It’s as if you were trying to open a jar with a stuck lid.” You keep twisting, and at first it doesn’t move, but eventually it gives way suddenly.”
Joachimiak claims that the antibiotic breaks its bond by activating a water molecule with zinc ions. ” He said that serial crystallography shows us exactly when the zinc causes the reaction. “You can observe it in real time.”
“The ability to resolve the dynamics of this particular class of molecules could have broad-reaching implications,” stated Mateusz Wilamowski, a researcher at Jagellonian University in Poland and a former postdoctoral researcher at the University of Chicago who also assisted with the research. He stated, “There are numerous other proteins like this that rely on mechanisms that are similar.” The molecule’s intermediate transitions, which we were able to see, have not been studied by anyone else.
According to Joachimiak’s explanation, regular protein crystallography does not permit the creation of these molecular movies because researchers can only collect a small number of images before destroying the crystal. He stated, “It’s truly a revolutionary technique that will have a significant impact on the manner in which we can observe and, ultimately, design better drugs.”
Wilamowski is also of the opinion that the findings will contribute to the development of intelligent drugs because future studies may combine the APS Upgrade with quantum mechanical calculations to enhance already existing molecules.
More information: M. Wilamowski et al, Time-resolved β-lactam cleavage by L1 metallo-β-lactamase, Nature Communications (2022). DOI: 10.1038/s41467-022-35029-3