For the purpose of creating the batteries that will power electronic devices, electric vehicles, and renewable energy grids, it is essential to comprehend why some materials work better than others at energy storage. A breakthrough made by Drexel University researchers could hasten the development of more effective energy storage technologies by making it possible to quickly pinpoint the precise electrochemical mechanisms operating in batteries and supercapacitors of various compositions.
The Drexel team’s approach, which was described in Nature Energy, combines two well-known scientific research techniques: one that analyzes chemical compounds’ compositions based on their capacity to absorb visible light, and another that gauges the electrical current flowing through energy storage systems like batteries and supercapacitors.
The researchers were able to more precisely monitor the movement of ions inside the devices by conducting these tests simultaneously, revealing the complex electrochemical process that controls the production of useful power.
“It’d be like opening your pantry door with your eyes closed and sniffing inside to see if there’s enough room for a few more cans of soup,”
John Wang, Ph.D., a postdoctoral research associate in the College of Engineering,
Obtaining a better view.
Danzhen Zhang, a doctoral student in the Department of Materials Science and Engineering in the Drexel College of Engineering and a co-author of the paper, stated that despite the field’s long history of research, we still do not fully comprehend the mechanisms of electrochemical processes in various energy storage systems.
We have a conceptual understanding of the electrochemical reactions involved, but it is still very challenging to quantify and meaningfully observe these complex electrochemical systems as they operate.”.
The difficulty comes from the fact that ions—the charged atomic particles that are packed into a device during charging and whose movement generates the electric current that allows it to power a device—cannot actually be seen. Both their size and their speed are inappropriate. The best thing that can be done by researchers is to rely on signals that show where they are most likely to be found—a sort of low-resolution atomic radar—by shooting particles at them and monitoring what bounces off.
It can be difficult to design electrodes properly to maximize energy storage area and enable orderly entrance and exit for the ions if one cannot see how ions are arranging themselves within, on top of, and between the energy storage compartments of the device.
John Wang, Ph.D., compared it to sniffing inside your pantry door while keeping your eyes closed to see if there is room for a few more soup cans. D., a co-author of the study and a postdoctoral research associate in the College of Engineering.
“At this time, it is still difficult to perform direct measurements and evaluate the operation of energy storage devices. We could design a structure that can hold a lot more ions if we could get a good look at the atomic structure and determine how and where the ions will fit. With the method we’ve developed, we think we can take those measurements and make those corrections.
Aiming to blend in.
At an electrode, ions can form in three different places: within its atomic layers, on its surface, or on top of other ions already present there.
Regarding the performance of batteries or supercapacitors, each of these configurations has advantages and disadvantages. More ions—and hence more energy—can be stored by intercalating, or entering, into the layers of the electrode material. A surface redox reaction, also known as an attachment and detachment process, allows for a rapid energy release. Additionally, an electrical double-layer reaction that allows solvent molecules to perch on top of an ion layer on the surface results in a slightly larger power discharge but with less energy.
To get a good idea of the dominant storage mechanism, researchers can measure how long it takes a storage device to discharge and then charge again or test the electrode material at the start and end of a discharge cycle.
An unsettling secret.
These energy storage mechanisms, however, might not always take place as ordered, discrete reactions, according to recent research. Numerous reactions are taking place with mixed or intermediate mechanisms. Therefore, for enhancing the performance of energy storage devices, it is crucial to correctly differentiate between them and comprehend them at their core.
Researchers will have a clearer understanding of all the reactions occurring and, more importantly, will be able to spot any parasitic side reactions that might impair the device’s functionality if ions in an electrode can be precisely quantified, tracked, and followed throughout its charge-discharge cycles.
With this knowledge, designers could more effectively customize the electrode materials and electrolytes to improve performance and reduce deterioration.
The two together are enlightening.
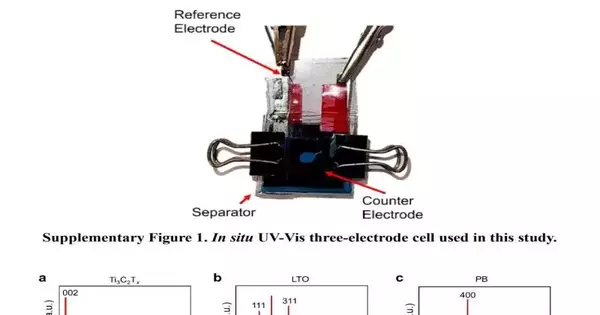
The new approach developed by the Drexel team provides a way to track the placement and motion of ions from electrolyte to electrode inside an energy storage device. Their method combines cyclic voltammetry (CV), which measures the electrical current during charge-discharge cycles, with ultraviolet-visible (UV-vis) spectroscopy, a technique for figuring out a compound’s chemical composition by how it absorbs light.
When the team used UV-vis spectroscopy to observe the electrochemical interaction in thin nanomaterial films of various electrode-electrolyte systems, they made a significant discovery. Although this application of UV-vis spectroscopy has not typically been made, the study’s electrode material’s thinness and transparency made it possible for UV-vis spectroscopy to characterize the electrochemical changes that occurred during charging and discharging.
The group captured spectral data using UV-vis at the same intervals as the electrochemical reactions to confirm their preliminary findings. During this process, they came to the conclusion that it might be feasible to synchronize visual UV-Vis spectral data with CV measurements of current, eliminating a level of uncertainty surrounding the electrochemical behavior they were trying to quantify.
The researchers were able to determine the timing of a specific reaction as well as how many electrons were being transferred during the reaction by comparing the signals from the two methods. This information is crucial for determining the type of electrochemical mechanism that is occurring.
The researchers created a plot known as a “UV-vis CV” curve by graphing the UV-vis data along with the CV measurements to link the findings. Due to the way electron transfer alters the way light passes through the material as well as shifts its electric current, each electrochemical mechanism—whether it is redox, partially redox, or electrical double-layer—plots as a distinct curve.
A line that plots in a roughly rectangular shape, for instance, would indicate electrical double-layer charging, whereas curves with sharp peaks would indicate a redox reaction.
They wrote, “The ‘UV-vis CV’ curves enabled us to identify a correlation between spectral changes and electrochemical processes, thereby facilitating the differentiation of electrical double-layer, pseudocapacitive, and intercalation-based battery-type redox processes.”. In a pseudocapacitive system, calibration of the oxidation state change allowed for the quantification of the number of electrons transferred during the reaction, much like in situ synchrotron X-ray absorption spectroscopy.
Enhancing the image’s clarity.
According to Danzhen, the correlation gave the team enough knowledge to comprehend how the electron structure of the electrode materials changed throughout cycling. And this is a more accurate measurement than those made using the more expensive and time-consuming techniques currently in use, like X-ray absorption or electron energy loss spectroscopy.
According to Danzhen, “by precisely matching or cross-referencing those measurements, we can eliminate the effects of parasitic reactions and improve the precision of our quantitative results.”.
A hypothesis that the mechanism governing the interaction between a water-in-salt electrolyte and a thin film electrode made of a two-dimensional, layered nanomaterial called MXene, which was discovered and studied at Drexel, was confirmed by the team after putting its method to the test.
In the past, researchers have used UV-vis to qualitatively distinguish different energy storage mechanisms, but they have never quantified redox activities, according to Danzhen. “By using optical signals to directly track changes in electrode materials, our UV-vis method for quantifying the electron transfer number effectively eliminates this effect. Derivative calculations within the UV-vis method also contribute to further eradicating errors that can occur when using traditional electrochemical characterization.”.
A more distinct future path.
The researchers propose that this approach could be a low-cost substitute for X-ray absorption spectroscopy—the equipment for which can cost more than $1 million—even though its current application would be limited to the transparency of electrode materials. Additionally, they note that it might make it easier to develop materials for energy harvesting, capacitive water deionization, electrochemical actuation, and energy storage.
“It is necessary to quickly assess and classify the electrochemical behavior of the materials being used in order to select the precise electrode materials and electrolytes from the myriad possibilities,” said Yury Gogotsi, Ph. D., in the College of Engineering, who is a distinguished university professor and a Bach scholar.
“Our method offers a quick and precise method for classifying how materials are interacting with ions in electrochemical systems, using equipment that is easily accessible. It might be possible to avoid a number of mistakes if we use this to plot our course toward better energy storage components and gadgets.
The group intends to carry out further research into more intricate electrochemical energy storage systems as well as test new electrolyte and electrode material combinations using its methodology.
More information: Xuehang Wang, In situ monitoring redox processes in energy storage using UV–Vis spectroscopy, Nature Energy (2023). DOI: 10.1038/s41560-023-01240-9. www.nature.com/articles/s41560-023-01240-9