Following quite a while of major logical and drug disclosure research, Alzheimer’s disease has remained enigmatic and hopeless, with an absolute minimum of restorative advancement. However, in another survey article in Nature Neuroscience, MIT researchers compose that by utilizing the new examination capacity of “single-cell profiling,” the field has quickly accomplished long-looked-for major areas of strength for both making sense of Alzheimer’s disease and accomplishing something significant about it. By examining this new proof, for example, the creators show that the sickness’ disturbances affect five primary areas of cell capability, or “pathways,” in every one of the five most significant synapse types.
Single-cell profiling advancements produce exhaustive estimations of hereditary action in individual cells, for example, levels of RNA, which is deciphered from DNA, so the cell’s capabilities and jobs in cerebrum science and disease pathology can be surveyed.Single-cell profiling innovations go beyond genome sequencing, which lists the DNA present in nearly every cell of an individual, by revealing how each cell is uniquely utilizing that standard set of instructions.
In concentrating on Alzheimer’s disease, researchers have been utilizing single-cell profiling to perceive how different synapses, for example, particular kinds of neurons, microglia, and astrocytes, act differently in illness compared with how they act in a healthy cerebrum.
“Therapeutic interventions may be able to correct abnormal cellular trajectories by identifying vulnerable cell types and the molecular programs that give rise to them. While many transcriptional modifications are cell-type specific, these changes may eventually converge on similar signaling pathways across cell types, which may provide targets for new therapeutic techniques.”
Mitch Murdock and Picower Professor Li-Huei Tsai, Director of MIT’s Picower Institute for Learning and Memory
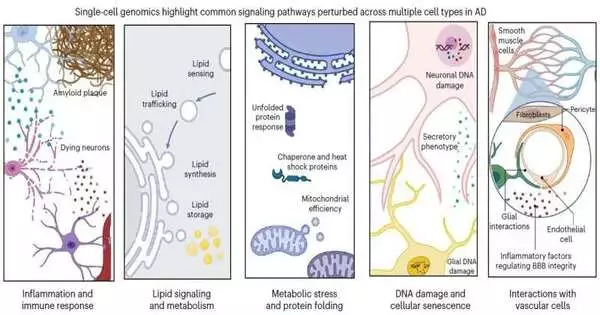
In the article, MIT Mind and Mental Sciences doctoral student Mitch Murdock and Picower Teacher Li-Huei Tsai, Overseer of MIT’s Picower Foundation for Learning and Memory and Maturing Cerebrum Drive, write that while single-cell profiling studies confirm that the illness’s awful effects are perplexing and extensive, there appear to be five pathways that become bothered in each of the five major cell types.Exploring these pathways may yield significant biomarkers of infection as well as significant targets for remedial intervention.The pathways are:
- Aggravation and a safe reaction
- Lipid (fat atom) flagging and digestion
- Metabolic pressure and protein collapsing
- DNA harm and cell senescence (maturing)
- Connections with cerebrum vasculature (veins)
For every one of these pathways in neurons, microglia, astrocytes, oligodendrocytes, and oligodendrocyte antecedent cells, Tsai and Murdock recognize explicit contrasts in quality guidelines, found in single-cell studies, that altogether happen in the cerebrums of Alzheimer’s patients or mouse models when contrasted with solid control tests.
For instance, Tsai and Murdock feature in excess of twelve qualities generally very familiar in lipid handling whose articulation is modified in different ways in different cells in the mind’s prefrontal cortex. For another model, they show that every one of the five cell types shows hindrances in DNA fixation, yet by different articulations of various qualities in each.
“By recognizing weak cell types and the atomic projects that bring about them, restorative mediations could turn around unusual cell directions,” Murdock and Tsai wrote in Nature Neuroscience. “While many transcriptional modifications are cell-type specific, these progressions may eventually combine on shared flagging pathways across cell types, addressing focuses for new beneficial methodologies.”
Certainly, the creators note, there is still a lot of work to be finished, both in refining and enhancing single-cell procedures and taking advantage of more current related open doors. The paper noticed various issues that should be painstakingly considered in delivering substantial single-cell profiling results, including where cells are examined in the mind for sequencing, from whom, and in what condition. Besides, it’s not generally clear to show what changes in quality articulation fundamentally mean for science, and it’s significantly more important to know whether a specific mediation, for example, targeting modified irritation pathways, will demonstrate protection and be compelling as a treatment.
Future bearings, in the mean time, could incorporate utilizing “spatial transcriptomics,” which estimates quality records in cells where they are arranged inside the cerebrum, as opposed to eliminating them for examination. Studies should be expanded to include more human examples in order to fully represent changing illness and segment contrasts.Datasets ought to be shared and coordinated, the writers should compose, and better correlations among human and mouse tests are important to more readily comprehend how well or not they cross over.
“Single-cell profiling works with a nuanced representation of the various cell processes afflicted in the promotion cerebrum,” Tsai and Murdock conclude. “These changed atomic projects assist with making sense of the disparity between solid maturing and mental degradation and feature cell-type-explicit sub-atomic projects engaged in promotion.” “Center flagging modules are disrupted across multiple cell types, and controlling disrupted cell states will prepare for new beneficial open doors.”
More information: Mitchell H. Murdock et al, Insights into Alzheimer’s disease from single-cell genomic approaches, Nature Neuroscience (2023). DOI: 10.1038/s41593-022-01222-2
Journal information: Nature Neuroscience