Many applications exist for nanoscale machinery, such as drug delivery, single-atom transistor technology, and memory storage. However, the machinery must be assembled at the nanoscale, which poses a significant challenge for researchers.
Quantum Interaction is a new artificial intelligence discipline that combines mathematics, physics, cognitive, and computer sciences. Quantum interaction applies insights, techniques, and models developed in quantum physics to new areas of informatics. Quantum informatics has so far been used in organizational dynamics, economics, information retrieval, linguistics, and biomedical informatics.
The ultimate goal for nanotechnology engineers is to be able to assemble functional machinery at the nanoscale piece by piece. In the macroscopic world, we can simply grab items and put them together. Single molecules are no longer impossible to “grab,” but their quantum nature makes their response to manipulation unpredictable, limiting the ability to assemble molecules one by one. This prospect is now a step closer to reality thanks to an international effort led by the Helmholtz Society’s Research Center Jülich in Germany, which includes researchers from the University of Warwick’s Department of Chemistry.
The delicate balance of interactions that keeps the molecule from collapsing is a true challenge for our quantum chemical simulation methods. The project not only taught us about the fundamental mechanisms that stabilize such unusual nanostructures, but it also assisted us in assessing and improving the capabilities of our methods.
Dr. Reinhard Maurer
An international team of researchers revealed the generic stabilization mechanism of a single standing molecule in the paper ‘The stabilization potential of a standing molecule,’ published in the journal Science Advances, which can be used in the rational design and construction of three-dimensional molecular devices at surfaces.
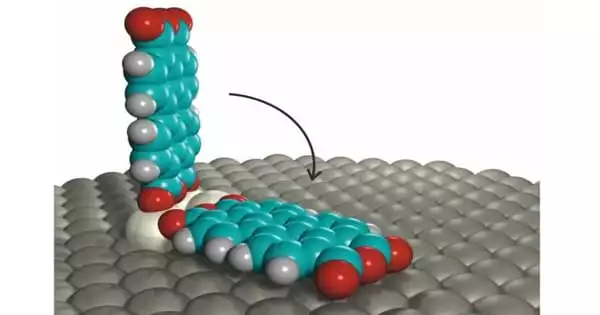
The scanning probe microscope (SPM) has brought molecular-scale fabrication closer to reality by allowing the rearrangement of atoms and molecules on surfaces, allowing the creation of metastable structures that do not form spontaneously. Dr. Christian Wagner and his colleagues used SPM to interact with a single standing molecule, perylene-tetracarboxylic dianhydride (PTCDA), on a surface to study its thermal stability and the temperature at which the molecule would cease to be stable and revert to its natural state, where it adsorbs flat on the surface. This temperature stands at -259.15 Celsius, only 14 degrees above the absolute zero-temperature point.
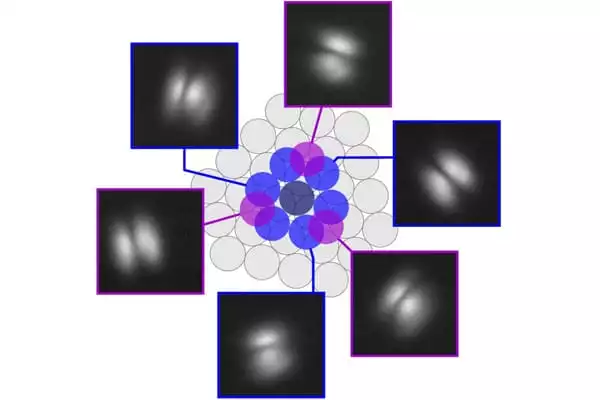
Quantum chemical calculations performed in collaboration with Dr. Reinhard Maurer of the Department of Chemistry at the University of Warwick revealed that the molecule’s subtle stability is caused by the competition of two strong opposing quantum forces, namely the long-range attraction from the surface and the short-range restoring force arising from the anchor point between the molecule and the surface.
Dr. Reinhard Maurer of the University of Warwick’s Department of Chemistry comments: “The delicate balance of interactions that keeps the molecule from collapsing is a true challenge for our quantum chemical simulation methods. The project not only taught us about the fundamental mechanisms that stabilize such unusual nanostructures, but it also assisted us in assessing and improving the capabilities of our methods.”
Dr. Christian Wagner of the Peter Grünberg Institute for Quantum Nanoscience (PGI-3) at Forschungszentrum Jülich has this to say: “To make technological use of individual molecules’ fascinating quantum properties, we must strike the right balance: they must be immobilized on a surface, but not too strongly, or they will lose these properties. Standing molecules are ideal for this. To determine how stable they are, we had to repeatedly stand them up with a sharp metal needle and time how long they survived at various temperatures.”
Now that the interactions that give rise to a stable standing molecule have been identified, future research can focus on creating better molecules and molecule-surface links to tune those quantum interactions. This can help to increase the temperature at which molecules can be switched into standing arrays, bringing them closer to workable conditions. This opens the door to the possibility of nano fabrication of machinery at the nanoscale.