A chromosome-level gathering of 14 snakes in a review driven by the Chinese Foundation of Sciences, China, has made a high-goal genomic reference for the investigation of snake development. The research team describes a wide range of previous discoveries made by their sequencing data in a paper that was published in Cell and titled “Large-scale snake genome analyses provide insights into vertebrate development.”
Researchers were able to construct a satisfactory draft of the entire genome for 14 snakes belonging to 12 distinct families by making use of the sequencers from PacBio and Oxford Nanopore Technology that have the capacity for long reads. The team then used platform sequencers from BGI and Illumina to get shorter, better-quality reads. The Hi-C read data were then used to put the reads together at the chromosome level.
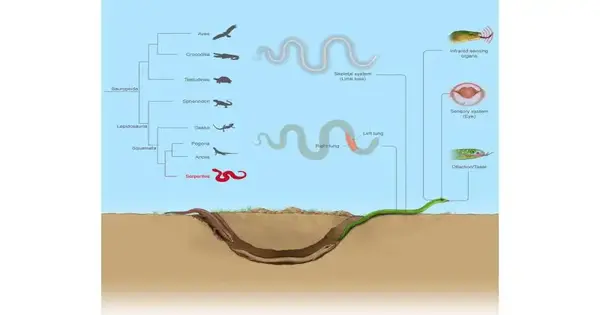
The data that the researchers gathered was then analyzed in conjunction with the genomes of a mouse, a crocodile, a bird, and a lizard that had already been sequenced. From various trait-related gene deletions, additions, and conservations, a picture of snake evolution was constructed.
The analysis’s estimated divergence times indicate that snakes originated approximately 118 million years ago and underwent rapid diversification following the mass extinction 65 million years ago.
All of the newly sequenced snake genomes had gene deletions that were specific to snakes, which was consistent with the signature features of a long body with no legs.
The shape of internal organs like the lungs is also affected by snakes’ long bodies. The left lung is commonly missing, remaining, or working, yet it is more modest than the right lung. The snakes lacked a dynein-coding gene, which is essential for the formation of left-right symmetries, and 13 lung development-related genes had insertions that could change how they regulate lung development.
Several lost coding genes that were related to photoreceptors showed changes in the visual system. In snake eyes, the expression of 15 genes related to photoreceptor cells was significantly elevated. The authors suggest that before returning to well-lit surface habitats, early snakes underwent a prolonged burrowing period in low light. The structure and function of the snake eyes would have reacted to surface light in this scenario by upregulating the conserved genes.
Pit vipers, pythons, and boas are the three snake species that have trigeminal nerves and specialized temperature-sensitive infrared-sensing organs. Non-coding genes were found to be repurposed convergently around active genes that control the cellular response to heat, as the analysis showed.
Gene loss is linked to snakes’ lack of hearing range and some sound perception, according to observations of hearing-regulating genes. At the same time, the specialization of the inner ear and low-frequency hearing may be related to the evolution of additional gene regulators. This change in hearing is also common in animals that burrow, which further suggests that they came from the underworld.
Beside the bits of knowledge into snake development and expansion, the top-notch information assortment has been disclosed and will without a doubt be a relied upon genomic reference hotspot for future examinations into reptilian science.
More information: Changjun Peng et al, Large-scale snake genome analyses provide insights into vertebrate development, Cell (2023). DOI: 10.1016/j.cell.2023.05.030