As indicated by new exploration, gadgets made of readily accessible oxide and carbon-based materials can deliver clean hydrogen from water over weeks.
The discoveries, co-drove by Dr. Virgil Andrei, a Research Fellow at St John’s College, University of Cambridge, with scholastics at Imperial College London, could assist with beating one of the main points of contention in sunlight-based fuel creation, where the flow of earth-bountiful light-engrossing materials is restricted through either their presentation or solidity.
Underexplored materials for light reaping
Hydrogen fuel will play a basic part in the change to full decarbonization and arriving at the UK’s objective of net-zero outflows by 2050. With most hydrogen presently provided from petroleum derivatives, analysts are currently attempting to track down ways of creating hydrogen more reasonably. One method for accomplishing this is to create gadgets that can reap daylight and split water to deliver green hydrogen.
While some light-retaining materials have been tried for green hydrogen creation, most debase rapidly when lowered in water. For instance, perovskites are the quickest developing materials with regards to light-collecting proficiency, yet they are unsound in water and contain lead. As a result of the risk of spillage, specialists have been attempting to foster without lead choices.
“Bismuth oxyiodide is an intriguing photoactive material with energy levels in the optimal places for water splitting. We established a few years ago that BiOI solar cells are more stable than those using cutting-edge perovskite light absorbers. We wanted to investigate if we could apply that stability to the creation of green hydrogen.”
Dr. Robert Hoye, Lecturer in the Department of Materials at Imperial College London
Bismuth oxyiodide (BiOI) is a non-harmful semiconductor elective which has been ignored for solar-oriented fuel applications because of its unfortunate strength in water. In any case, in light of past discoveries into the capability of BiOI, scientists chose to return to the material’s commitment for the development of green hydrogen.
Dr. Robert Hoye, Lecturer in the Department of Materials at Imperial College London, made sense of this: “Bismuth oxyiodide is an entrancing photoactive material that has energy levels at the right situations for water parting.” A couple of years prior, we demonstrated that BiOI sunlight-based cells are more steady than those utilizing cutting-edge perovskite light safeguards. We needed to check whether we could make an interpretation of that dependability to green hydrogen creation.
Teacher Judith Driscoll, Department of Materials Science and Metallurgy, University of Cambridge, says that “we have been chipping away at this material for quite a while, because of its wide-running likely applications, as well as its effortlessness of manufacture, low poisonousness, and great steadiness.” “It was perfect to consolidate the abilities of the different exploration groups across Cambridge and with Imperial.
Leap forward in sun-powered fuel creation
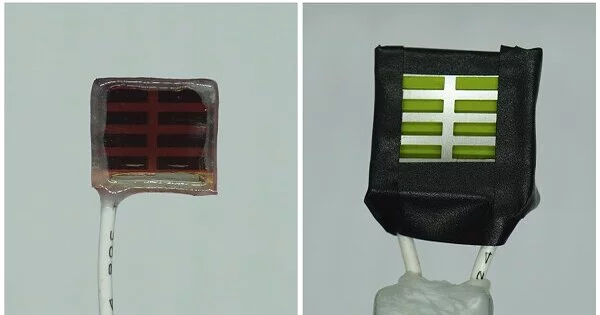
The group of analysts made gadgets that imitated the regular photosynthesis process happening in plant leaves, aside from producing hydrogen rather than sugars. These fake leaf gadgets were produced using BiOI and other maintainable materials, harvesting daylight to deliver O2, H2 and CO.
Scientists figured out how to increase the dependability of these fake leaf gadgets by embedding BiOI between two oxide layers. The powerful oxide-based gadget structure was additionally covered with a water-repellent graphite glue, which forestalled moisture invasion. This delayed the strength of the bismuth oxyiodide light-engrossing pixels from minutes to several months, including the time the gadgets were left away.

This is a critical finding that changes BiOI into a feasible light collector for stable green hydrogen creation.
“These oxide layers work on the capacity to deliver hydrogen contrasted with independent BiOI,” said Dr. Robert Jagt (Department of Materials Science and Metallurgy, University of Cambridge), one of the co-lead creators.
Specialists further found that counterfeit leaf gadgets containing different light-collecting regions (called “pixels”) exhibited a better execution than traditional gadgets with a solitary bigger pixel of the same complete size. This finding could make the scale-up of novel light reapers a lot more straightforward and quicker for economical fuel creation.
Dr. Virgil Andrei, a co-lead creator from the Department of Chemistry in Cambridge, makes sense of this: “Regardless of whether a few pixels are flawed, we had the option to disengage them, so they don’t influence the rest.” This implied we could support the exhibition of the little pixels on a bigger region. This expanded presentation empowered the gadget to deliver hydrogen as well as less CO2 to blend gas, a significant middle of the road in the modern combination of synthetic substances and drugs.
Planning ahead
The discoveries exhibit the potential for these new gadgets to challenge the presentation of existing light safeguards. Better methods for making BiOI counterfeit leaf gadgets more stable can now be intended in other novel frameworks, assisting with commercialization.
“This is an interesting turn of events. Right now, hardly any sun-oriented fuel frameworks show sound qualities that are viable for true applications. “With this work, we make a step forward towards laying out a roundabout mileage,” said Prof. Erwin Reisner (Department of Chemistry, Cambridge), one of the related creators.
The discoveries have been published in the journal Nature Materials.
More information: Virgil Andrei et al, Long-term solar water and CO2 splitting with photoelectrochemical BiOI–BiVO4 tandems, Nature Materials (2022). DOI: 10.1038/s41563-022-01262-w