A protein that plans DNA for replication likewise prevents the replication interaction from running wild, as per another concentrate by Weill Cornell Medicine specialists. The work, distributed Jan. 5 in Sub-atomic Cell, settles a secret that has long bewildered scientists.
The cells of people and any remaining higher organic entities utilize a perplexing arrangement of designated spots and “permitting” proteins to guarantee that they recreate their genomes unequivocally once prior to partitioning. In anticipation of cell division, the permitting proteins connect to explicit areas in the DNA, designating them as replication beginnings. At the point when the DNA blend period of the cell cycle starts, replication starts just at those authorized destinations and just starts, or “flames,” once, as indicated by the ongoing model.
However, that model was feeling the loss of an essential point. “The factor that is allowing this to happen is simply debased after these replication starting points have terminated,” said senior creator Dr. Tobias Meyer, the Joseph Hinsey Teacher in Cell and Formative Science at Weill Cornell Medicine.”On a basic level, the cell could stack these permitting machines onto DNA that is as of now reproduced, thus, rather than two duplicates, you’re getting three or four duplicates of that fragment of the DNA, and these cells would be supposed to lose genome respectability and bite the dust or become carcinogenic.”
“The cell could theoretically load these licensing machines onto DNA that has already been copied, so, instead of getting two copies, you’re getting three or four copies of that portion of the DNA, and these cells would be predicted to lose genomic integrity and die or become malignant.”
Senior author Dr. Tobias Meyer, the Joseph Hinsey Professor in Cell and Developmental Biology
Sorting out how cells stay away from that destiny has been interesting. “We should have been concentrating on occasions in the main minutes of the DNA blend period of the cell cycle, so it’s an extremely transient period,” said first creator Nalin Ratnayeke, an alumni understudy who dealt with this task both at Stanford College and at Weill Cornell Medication in Dr. Meyer’s lab.
The lab moved to Weill Cornell Medicine in 2020. To address this difficult exploratory issue, Ratnayeke used PC-assisted microscopy to screen a large number of developing cells while keeping the repeating cells in the demonstration and dissecting the activities of their permitting and replication factors.
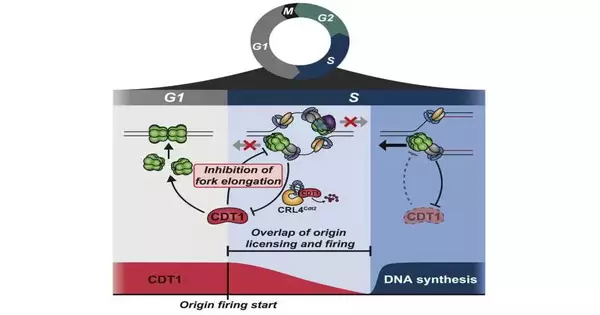
The work uncovered that a notable permitting factor, CDT1, not just licenses a fragment of DNA to turn into a replication beginning, yet in addition goes about as a brake for DNA replication, forestalling a fundamental replication catalyst called CMG helicase from working. To begin incorporating DNA, the cell’s catalysts should initially separate CDT1.
Recently proposed components for planning this change from the authorizing period of the phone cycle to the terminating period of the cell cycle have relied upon repressing permitting factors, said Ratnayeke, adding that “the instrument that we distinguished here is really the inverse… the authorizing factor CDT1 itself is forestalling the movement of DNA combination.”
To affirm their outcomes, the researchers teamed up with partners at the Clinical Exploration Board in Cambridge, UK, who found that the inhibitory component can be reiterated in an improved on framework that repeats the whole DNA union cycle with purged parts in a test tube.
“That permitted us to reconstitute every one of the parts for DNA amalgamation and to demonstrate that CMG helicase is straightforwardly restrained by CDT1,” said Dr. Meyer, who is likewise a teacher of organic chemistry and an individual from the Sandra and Edward Meyer Malignant Growth Place at Weill Cornell Medicine.
Because failures in replication permitting can cause cells to die or become carcinogenic, the findings provide a new perspective on cell health and disease. “Future work to distinguish unthinkingly what’s the deal with Cdt1 hindrance will give more noteworthy understanding into the biophysics of how CMG helicase works and will pinpoint explicit areas of this perplexity that can be treated with drugs,” Ratnayeke said.
More information: Nalin Ratnayeke et al, CDT1 inhibits CMG helicase in early S phase to separate origin licensing from DNA synthesis, Molecular Cell (2023). DOI: 10.1016/j.molcel.2022.12.004