When Courtney “CJ” Johnson pulls up footage from her Ph.D. thesis, it appears as if she is watching an attempted break-in on a home surveillance camera.
The gatecrasher approaches his target without stepping inside, looking for a way in.Yet, this gatecrasher isn’t your ordinary robber. It’s an infection.
The recording, shot north of two minutes by pinpointing its area 1,000 times per second, shows a small infection molecule, many times smaller than a grain of sand, reeling and swaying among tightly packed human digestive cells.
Briefly, the infection connects with a cell and skims along its surface, but doesn’t stick prior to jumping off once more. Assuming this were a genuine home break-in, Johnson says, “This would be the part where the thief has not broken the window yet.”
An infinitesimal video shows an infection (purple track) as it tracks down its direction to the outer layer of human gastrointestinal cells (green). Credit: The Welsher Lab, Duke College
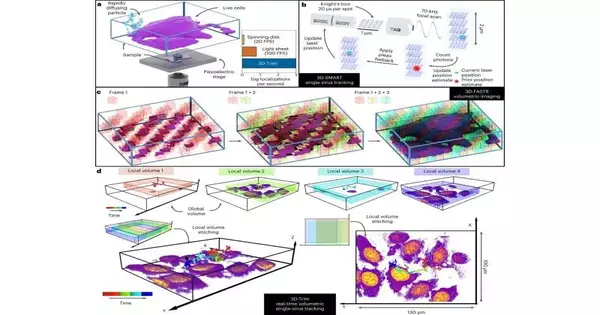
Johnson is essential for a Duke College group led by partner science teacher Kevin Welsher. Along with Welsher’s postdoctoral partner Jack Exell and partners, they have concocted a method for catching a constant 3D film of infections as they approach their cell targets. Their examination is distributed today in the journal Nature Strategies.
We consistently breathe in, ingest, and absorb a large number of infections.The vast majority of them are innocuous, yet some of them—for example, the infections that cause this season’s virus, or Coronavirus—can make us debilitated.
showing the working guidelines for 3D-TrImMovement succession starts with an outline of an exploratory arrangement in which a warmed example containing infection-like particles (VLP) and live cells is mounted on a piezoelectric stage with an objective focal point shared by both following and imaging magnifying lens sources. This outline is trailed by a movement of 3D-Brilliant continuous following, showing how a couple of Electro-Optic Diverters (EOD) make a sidelong Knight’s Visit matrix design, followed by the utilization of a tunable acoustic slope (label focal point) to check a central reach above and underneath the focal point of the central volume. A last movement shows the rule of 3D-FASTR point-check imaging.
Disease begins when an infection ties to and enters a cell, where it commandeers the cell hardware to make duplicates of itself. Yet before it can break in, an infection needs to arrive at the cell first, Johnson said.
That frequently implies overcoming the defensive layer of cells and bodily fluid that lines the airways and the stomach—oone of the body’s most memorable lines of safeguard against disease.
VSV-G is investigating the extracellular network, which is connected with Fig. 2a, b. A 4D dataset covering 10 nearby volumes at 16 FPV was used to create a 3D recreation of ongoing VSV-G VLP direction in the extracellular lattice of live GM701 cells (stained with F-actin mark SiR650-actin).The direction (162 seconds) is divided into 25 fragments per second (25 casings per subsequent when playback rate is 1), and variation is planned by time.The advancement bar shows how the direction is additionally classified. (1) Free dissemination period (playback rate: 2 Hz): 0–14 s, 18–38 s, 44–62 s, 70–108 s (2) Skimming period (playback rate: 1): 14–18 s; 38–44 s; 62–70 s; 108–122 s. (3) Interval (playback rate: 2): 122-162 sCircle addresses the VLP position in the current edge (reviving rate is constant with direction, or at least 25 FPS at 1 playback rate).Picture volumes shaped by the most extreme power projection from nearby volumes gained north of 16 casing times over the long haul.In a, cells are variety coded by imaging power, while in b, cells are variety coded based on the distance of the infection from the cell surface. Boards an and b share a similar direction variety scale, camera point, and camera way; nonetheless, an is amplified in contrast to b.
The analysts needed to comprehend how infections break these bleeding-edge guards. “How do infections explore these mind-boggling boundaries?” Welsher said. However, these fundamental early minutes before disease begins have been troublesome for quite some time in the event that they are not difficult to watch with existing microscopy strategies, he added.
Some portion of the explanation is that infections move a few significant degrees quicker in the unconfined space outside the cell compared to its packed interior. To make things much trickier, according to an imaging viewpoint, infections are many times smaller than the cells they contaminate.
“That is the reason this is a difficult issue to study,” Johnson said. Under the magnifying lens, “it’s like you’re attempting to snap a photo of an individual remaining before a steep rise.” “You can’t capture the entire high rise and the subtleties of the individual before it in a single photograph.”
So the group fostered another technique called 3D Following and Imaging Microscopy (3D-TrIm), which basically joins two magnifying lenses into one. The primary magnifying lens “locks on” to the quick infection, clearing a laser around it a huge number of times each second to work out and refresh its situation. As the infection bobs and tumbles around in the soupy outside of the cell, the magnifying lens stage constantly changes to keep it in the center.
While the main magnifying lens tracks the infection, the subsequent magnifying instrument takes 3D pictures of the encompassing cells. The combined effect, according to Welsher, is similar to exploring with Google Maps: it not only shows your current location as you drive, but it also shows the territory, landmarks, and general lay of the land, all in 3D.
“In some cases, when I present this work, individuals ask, “Is this a computer game or a reproduction?” “,” said Johnson, presently a postdoctoral partner at the Howard Hughes Clinical Establishment at Janelia Research Grounds. “No, this is the sort of thing that came from a genuine magnifying lens.”
With their technique, the specialists can’t simply, say, watch a sound individual take in infection particles from a contaminated individual’s hack or wheeze. As far as one might be concerned, they need to connect a unique fluorescent name to an infection before they can follow it—what the magnifying instrument follows is the development of the sparkling spot. Furthermore, at the moment, they can track an infection for a few seconds before it fades.
“The greatest test for us currently is to deliver more brilliant infections,” Exell said.
Yet, Welsher said he trusts the strategy will make it conceivable to follow infections in real life past the coverslip and in more reasonable tissue-like conditions where contaminations first grab hold.
“This is the genuine commitment of this strategy,” Welsher said. “We believe that is something we have the likelihood to do now.”
More information: Courtney Johnson et al, Capturing the start point of the virus–cell interaction with high-speed 3D single-virus tracking, Nature Methods (2022). DOI: 10.1038/s41592-022-01672-3
Journal information: Nature Methods