Another review from the University of Miami and University of Nebraska- Lincoln has overturned many years of tenets about a protein whose change or glitch can set off lethal illnesses right off the bat throughout everyday life. The examination group, which included Nebraska’s Jonathan Dietz and Javier Seravalli, detailed its discoveries in the journal Nature Communications.
That protein, cytochrome c oxidase, lives in mitochondria, going about as a door into the cell organelles most popular for producing energy that drives a wide range of life-supporting tasks. Cytochrome c oxidase is also one of the last performers in a Rube Goldberg-esque procession of connections that transport and eventually convert biochemical currency — oxygen and glucose — into the energy-storing particle known as ATP.
By the same token, the gathering of cytochrome c oxidase itself is not really a basic interaction. Truth be told, organic chemists have gone through years sorting out how the huge protein gets sorted out.
“It’s truly modern hardware,” said Oleh Khalimonchuk, Susan J. Rosowski Professor of natural chemistry at Nebraska and a main co-creator of the review.
“It turns out that the notions are not quite the same in human cells as they are in studies of bacteria and yeast. In terms of how these factors are acting, I believe this essentially challenges a decades-old paradigm.”
Oleh Khalimonchuk,
Natural chemists first explored the protein in microbes, yeast, and other single-celled life forms that advanced cytochrome c oxidase well before people did. However, the complexity of both human cells and the protein, which has more than twelve subunits, has complicated attempts to survey its accumulation in those cells.Until now, however, analysts have assumed that the gathering of the protein’s reactant center works out in people much as it does in microorganisms.
Driven by Miami’s Antoni Barrientos and Eva Nvltová, the Hurricane-Husker group embraced the tiring errand of erasing, individually, in excess of twelve qualities that direct the creation of proteins and different atoms accepted to gather the catalyst in people. By examining the consequences of every erasure, the analysts figured out how to recognize the atomic MVPs on the mechanical production system, portray their commitments to that gathering, and decide their requests for tasks with close-to-unusual accuracy.
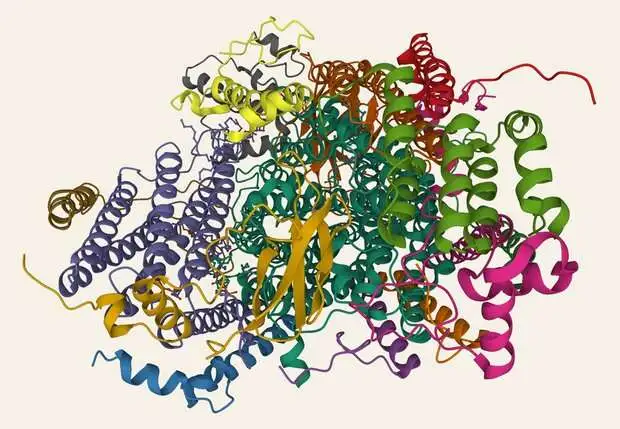
A delivery of cytochrome c oxidase.
Some of what they found opposed the long-acknowledged writing of the field.
“The manner in which the ideas have been spread out in microbes and yeast concentrates ends up not being the very same in human cells,” Khalimonchuk said of the protein-gathering parts. “I think this essentially breaks a decades-old worldview as far as how these elements are acting.”
One of the chief shocks came as COX11, a gathering helping protein so basic in yeast that without it, Khalimonchuk said, “Those mitochondria are down and out.” The group confirmed that COX11 collaborates with other proteins in human cells to chaperone copper iotas across a phone and settles them in two center subunits of cytochrome c oxidase.From that point, the copper iotas assist in setting off the creation of energy-putting away ATP by tolerating electrons and passing protons into a mitochondrion.
However, when the analysts took out the quality liable for COX11, they found that the subsequent cells actually prevailed with regards to gathering around 15% as many cytochrome c oxidases as they normally do, keeping up with generally 60% of their typical ATP creation. The group later resolved that human cells missing COX11 can hit up different proteins that — in spite of the below-normal — can really sub in for it. Also, when the group controlled cells to produce a greater amount of the subbing in protein known as PET191, the COX11-less gathering of cytochrome c oxidases hopped from 15% to 40%.
“It settled a problem on the grounds that a ton of changes in the gathering elements of cytochrome oxidase are connected to illnesses in people,” Khalimonchuk said. “There are lots of natural illnesses, yet never have they been accounted for with a change in COX11—dissimilar to whatever other element that we’ve been managing. This was so odd. Yet, presently, this information really makes sense of why since it’s somewhat unnecessary.
Yet, Khalimonchuk and his partners likewise presumed that COX11, which gets together with specific proteins until those proteins are prepared to chaperone copper to the compound’s center subunits, goes about as an administrative shield against the drawn-out development of harmful particles. That is especially significant, he said, given the touchy mix of oxygen and electron-rich atoms frequently twirling around mitochondria.
“You can consider oxidase a delayed bomb, since you have everything that is ready for sure fire response — tolerating electrons, responding with oxygen, etc,” Khalimonchuk said. “So everything must be tweaked and come into place (brilliantly). There’s something off about the event that — assuming you have some ill-advised gathering, some vagrant subunits, something presented to a climate that it shouldn’t be — that is truly risky. They will start up immediately, and that has a few pretty negative ramifications for a cell.
“Part of the explanation why these illnesses are accepted to be so awful and extreme is a result of these elements in cytochrome oxidase.”
In this regard, Khalimonchuk believes that studying the details of cytochrome c oxidase will be critical for better diagnosing and eventually treating the evil illnesses that its malfunction can cause.One uncommon yet wrecking model, Leigh disorder, besets the sensory systems of babies, who by and large endure something like a couple of years.
“There’s an entire range of innate illnesses,” Khalimonchuk said. “They’re all beginning stages, since you want to breathe (and produce ATP) basically at every turn. “Mitochondrial illnesses are awful as a rule, yet these are especially terrible.”
In making sense of the value of mitochondria-centered discoveries, Khalimonchuk referred to recent research showing that managing oxygen for patients with extreme mitochondrial illnesses—a typical strategy in trauma centers—is really negative.
That by itself addresses progress, Khalimonchuk said. Yet, moving from correctives and proposals to possibly life-saving meds will require that organic chemists keep brushing the fine print from the guidance manuals of cytochrome c oxidase and mitochondrial hardware like it. That implies cooperation, he said, and a readiness to take on the “long trudge” of the laborious yet vital work.
“We trust that there will be more subsequent meet-ups, not only by us, to far superior grasp this entire cycle,” Khalimonchuk said, “and perhaps discover a few other lacking parts.”
More information: Eva Nývltová et al, Coordination of metal center biogenesis in human cytochrome c oxidase, Nature Communications (2022). DOI: 10.1038/s41467-022-31413-1
Journal information: Nature Communications