A group led by University of Minnesota Twin Cities biomedical specialists has fostered a generally open application that can reproduce complex sub-atomic cooperations, which will permit scientists to configure better therapies for illnesses like malignant growth and COVID-19.
The paper expands upon a review the scientists distributed in 2019. They’ve extended the innovation to recreate considerably more perplexing atomic communications, made the application simple for non-specialists to utilize, and applied their discoveries to reveal insight into how the SARS-CoV-2 infection taints the body.
The review is published in Nature Communications, and the application, called MVsim, is openly accessible to different specialists on GitHub.
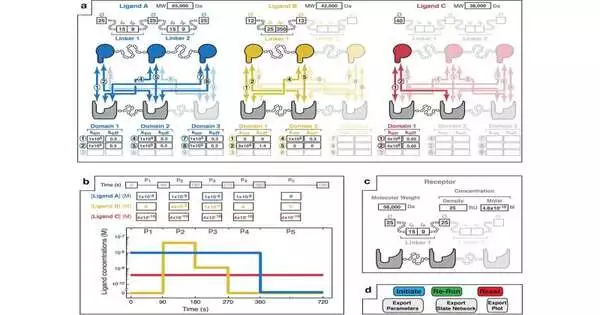
The test system predicts the strength, speed, and selectivity of multivalent associations, which include atoms that have different restricting locales and can be utilized to foster prescriptions for infections, especially malignant growth and COVID-19.
“Multivalent communications are truly significant in normal natural frameworks, and they are currently beginning to be imaginatively taken advantage of for making new restorative medications that influence their remarkable restricting properties,” said Casim Sarkar, senior creator of the paper and a teacher in the University of Minnesota Department of Biomedical Engineering.
“Multivalent interactions are extremely important in natural biological systems, and they are currently being creatively utilized for the development of new therapeutic medicines that leverage their unique binding capabilities.”
Casim Sarkar, senior author of the paper and a professor in the University of Minnesota Department of Biomedical Engineering.
“With multivalent medications, you can, on a basic level, target cells explicitly, such that is impractical with standard, monovalent medications, yet there are numerous factors to consider in their plan and a large part of the work in the field to date has been finished through exploratory experimentation,” Sarkar added. “Presently, utilizing MVsim, we’re ready to make great forecasts that can be utilized to all the more sanely plan such therapeutics.”
Numerous malignant growth drugs are tied to cancer cells as well as to cells they aren’t intended to target, which frequently has undesirable secondary effects for the patient. By improving the particularity of multivalent connections utilizing MVsim, scientists can configure sedates that all the more explicitly focus on the cells in growth while restricting them to different cells in the body.
Another model is the SARS-CoV-2 infection. Researchers realize that the infection is advancing to more readily contaminate our cells and dodge our safe frameworks, yet the atomic instruments behind how the infection does this is moderately obscure. Utilizing their MVsim innovation, the University of Minnesota specialists had the option to investigate this cycle more top to bottom, revealing the rates at which individual restricting spaces inside the infection’s multivalent spike protein switch between a cell-tainting state and an insusceptible sidestepping state.
“We basically have a computational magnifying lens that permits us to look in the engine and see what multivalent proteins, for example, the SARS-CoV-2 spike protein, are doing at a sub-atomic level,” Sarkar made sense of. This degree of sub-atomic detail is difficult to detect with an actual investigation. One of the genuine powers of MVsim is that we can not just find out about how these frameworks work, yet we can likewise involve this apparatus to plan new multivalent connections for illnesses like malignant growth and COVID-19. “
The specialists have proactively distinguished possible ways of restricting the infectivity of current and future SARS-CoV-2 variations, which they intend to test soon.
More information: Bence Bruncsics et al, MVsim is a toolset for quantifying and designing multivalent interactions, Nature Communications (2022). DOI: 10.1038/s41467-022-32496-6
MVsim app: GitHub
Journal information: Nature Communications