As significant as the gas powered motor has been for cultural advancement, it is likewise a significant supporter of contamination, harming human wellbeing and fossil fuel byproducts that assist in driving the environmental emergency. Nearly 30% of U.S. fossil fuel byproducts come from transportation, and 95% of transportation utilizes petroleum products.
Powering vehicles with hydrogen energy units, which emit only water fumes, is one component of a potential cure. Nonetheless, this manageability arrangement has an amusing, worked-in viewpoint that is impractical: The impetuses important for drawing power from hydrogen include uncommon and costly metals like platinum. In the amounts required for the current innovation, widespread acceptance would necessitate quantities of these metals that exceed what humanity can supply.
A new report in Nature Nanotechnology led by a UCLA teacher might address a defining moment. The scientists devised a methodology that enabled them to meet — and exceed — the Department of Energy’s aggressive targets for high impetus execution, high security, and low platinum utilization. Their record-breaking innovation utilized miniscule gems of a platinum-cobalt compound, each implanted in a nanopocket made of graphene, which is depicted as a two-layered material since it contains a layer of carbon one iota thick.
“This discovery was made by chance. We knew we were on to something that could stabilize tiny particles, but we didn’t anticipate it to work that well.”
Yu Huang, professor and chair of the Department of Materials Science and Engineering
Contrasted with the rigid DOE norms for impetuses—neglected till now—the creators’ graphene-wrapped amalgam yielded uncommon outcomes:
- Multiple times more reactant action
- 65% more power.
- Around 20% more reactant action at the normal finish of the energy unit’s life.
- Approximately 35% less loss of force subsequent to testing that mimics 6,000 to 7,000 hours of purpose, beating the objective of 5,000 hours.
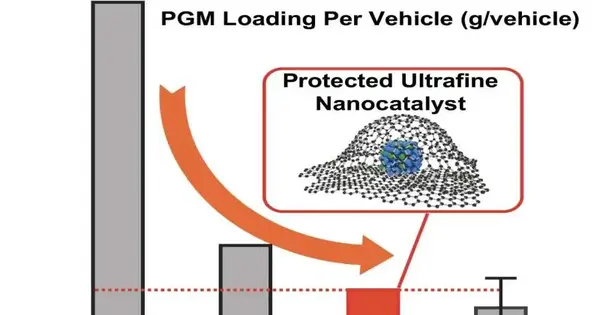
- Each vehicle contains nearly 40% less platinum.
“This has never been finished,” said related creator Yu Huang, teacher and chair of the Department of Materials Science and Engineering at the UCLA Samueli School of Engineering and an individual from the California Nano Systems Institute at UCLA. “This disclosure included some luck. We realized we were onto something that could make more modest particles stable, yet we didn’t anticipate that it should work this well. “
Today, a large part of the world’s total stock of platinum and comparable metals is utilized for exhaust systems in vehicles controlled by petroleum products, a part that makes their discharges less toxic. Somewhere between 2 and 8 grams of platinum are required per vehicle. By correlation, current hydrogen energy unit innovation utilizes around 36 grams for each vehicle.
At the lowest heap of platinum tried by Huang and her group, every hydrogen-fueled vehicle would require just 6.8 grams of platinum.
So how did the analysts get more power out of less platinum? They split the platinum-based impetus up into particles of a normal of 3 nanometers in length. A nanometer is one-billionth of a meter, and the nanoparticles were small to the point that it would take in excess of 30,000 starts to finish to traverse the thickness of a solitary piece of paper. More modest particles mean more surface area, and more surface area implies more land where reactant action can happen.
There’s a trick, however, that has hindered past endeavors to get better execution by going a little with hydrogen energy unit impetuses. All alone, smaller particles are likewise undeniably less solid, since they will generally peel away from a surface or group together into bigger particles.
Huang and her colleagues approached this limit by encasing their impetus particles in the 2D material graphene. Contrasted with mass carbon as usually found in coal or pencil lead, such thin layers of carbon have amazing limits, directing power and intensity effectively and showing strength multiple times that of steel at comparable thickness.
Their platinum-cobalt amalgam was decreased to particles. Prior to being coordinated into an energy unit, the particles were encircled by graphene nanopockets, which acted as a kind of anchor to hold the particles back from moving — vital for the degree of strength required in business vehicles. Simultaneously, the graphene considered a small hole, of around 1 nanometer, around every impetus nanoparticle, which implied that key electrochemical responses could happen.
“It’s kind of natural,” Huang said. “Assuming you set a limit for the molecule that permits the response to continue yet limits the molecule there, it will determine the strength issue, which is anyway extremely testing to accomplish at such a limited scope.”
This most recent development follows a new cooperative review driven by Huang that created a model for foreseeing the reactant action and strength of a platinum-based compound that can be utilized to direct the plan of impetuses — the first of its sort. She and her group are attempting to make an interpretation of their trial results into a useful innovation that can be taken to the market and, ideally, add to efficient power energy and manageability endeavors.
More information: Zipeng Zhao et al, Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions, Nature Nanotechnology (2022). DOI: 10.1038/s41565-022-01170-9
Journal information: Nature Nanotechnology