Decreasing energy utilization is one of the prerequisites for adaptable electrochemical energy change and electrosynthesis, including the electrochemical reduction of CO2 (ECR) into synthetic compounds.
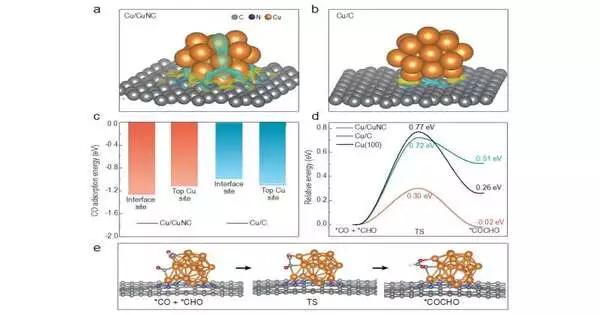
This review, distributed in the diary Public Science Survey and driven by Prof. Jin-Melody Hu (Establishment of Science, Chinese Foundation of Sciences) and Prof. Jinlan Wang (School of Physical Science, Southeast College), targets lessening the overpotential for ECR. The hypothetical estimation results were performed right off the bat, proposing that the lopsided electronic design on the Cu/CuNC point of interaction could essentially upgrade the adsorption of the CO transitional and decrease the response energy obstruction of the C coupling, in this way working on the selectivity of the ethanol at a low overpotential.
The scientists hence blended such an impetus with a high thickness of Cu/CuNC interfacial destinations by the in-situ electrochemical decrease of exceptionally stacked CuNC SACs. Ex-situ spectroscopic portrayal results proved that the nano-sized Cu nanoparticles (3.6 nm) were shaped and encircled by nitrogen (N).
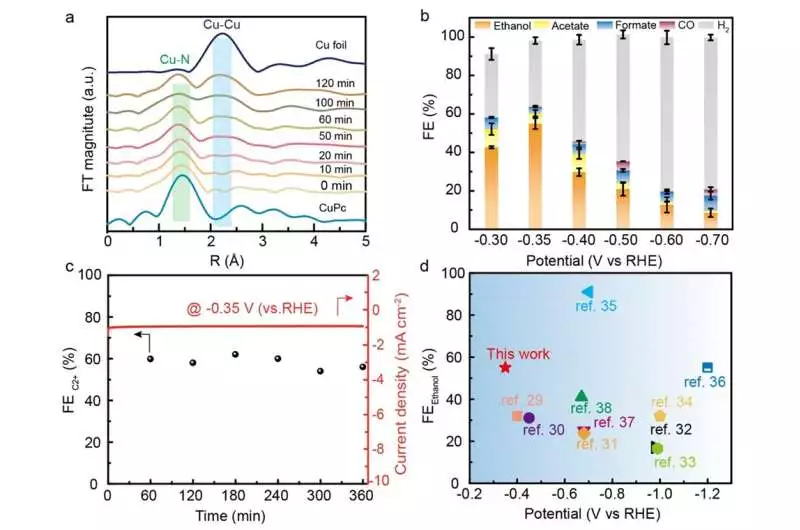
(a) Cu K edge EXAFS spectra for emergency room Cu/CuNC at various electrochemical decrease times at – 0.30 V; (b) Potential-subordinate FEs for various items; (c) Long haul strength at – 0.35 V; and (d) Execution examination.
In-situ spectroscopic portrayal results uncovered the arrangement interaction of the Cu/CuNC interfacial destinations and the conjunction of zero-valence Cu and Cu-N-C locales. The ECR tests revealed that the trama center Cu/CuNC impetus demonstrated an incredible faradic effectiveness of 60.3% for C2+ (55% for FEethanol) at a low capability of – 0.35 V versus RHE, outperforming most previously announced Cu-based impetuses.
Likewise, the systematical control tests demonstrated that the impetuses without Cu/CuNC interfacial locales conveyed an immaterial ethanol creation rate, supporting the basic job of the Cu/CuNC interfacial destinations in advancing C coupling at a low overpotential. These discoveries gave new bits of knowledge and appealing ways to deal with making multisite interface as exceptionally effective reactant places for elevating ECR to C2+ items at low energy cost.
More information: Yan Yang et al, In-situ Constructed Cu/CuNC Interfaces for Low-Overpotential Reduction of CO2 to Ethanol, National Science Review (2022). DOI: 10.1093/nsr/nwac248