Researchers say a secret about how a synthetic compound found in nature could be blended in the lab might have been revealed— a leading edge that could open new improvements in medication.
Researchers from colleges and examination foundations in Scotland and Germany are behind the revelation, presently distributed in the journal Nature Science. The paper shows interestingly the way in which three proteins are critical to the creation of alkaloid compounds called crocagins.
Alkaloids gotten from normal sources have provided us with many indispensably significant prescriptions; morphine is maybe the most renowned model.
Pyrroloindolines are alkaloids that are normally delivered by certain sorts of microbes, parasites, and plants, as well as through the skin emissions of certain kinds of frogs. Past examination has recommended that they have strong bioactive properties, which could make them valuable as anti-infection agents, antivirals, and even malignant growth medicines.
The work could prepare specialists to utilize the crocagin “platform”—the particles’ center construction — as a beginning stage to look for new medications in light of the center design of pyrroloindolines.
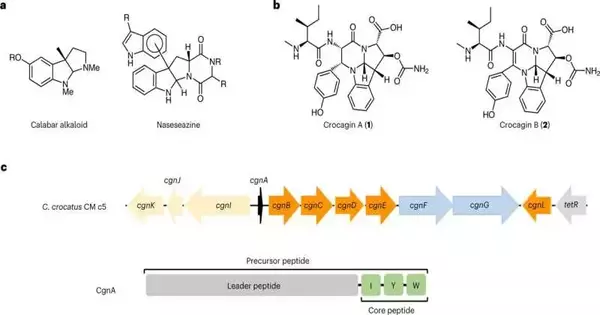
Crocagins are created from a peptide that has been made by the ribosome, the same way most proteins in cells are made, and this peptide then, at that point, gets changed by specific catalysts.
These kinds of regular items, called ribosomally blended and post-translationally adjusted peptides (RiPPs), are increasingly important to analysts in applications including medication and biotechnology.
Progress in quality sequencing and altering has made it conceivable to design RiPPs to outfit those exceptional properties for new improvements across a scope of ventures securely.
In the paper, the scientists depict how they unwound the biochemical pathway that produces crocagins and showed how the center construction of the alkaloid crocagin can be blended from a forerunner peptide, CgnA, by three compounds, CgnB, CgnC, and CgnE, in one stage.
They likewise utilized bioinformatic examination strategies to track down the hereditary outlines to make comparative particles in different microbes.
Teacher Jesko Koehnke, of the College of Glasgow’s School of Science, drove the exploration and is the paper’s compare and contrast creator. He said, “It is extremely energizing to find how we can transform a peptide into this sort of alkaloid, utilizing the normal devices that development has given, particularly in light of the fact that it opens up the chance of finding related particles with bioactivities that could be valuable in new applications.
“Pyrroloindolines are additionally difficult to combine in the lab, and ideally our bits of knowledge will add to the various approaches to making these particles.
“This is a thrilling disclosure, and one that took quite a while to really take shape as we attempted to more deeply study the biochemical pathway engaged with the cycle. It could never have been feasible to translate this pathway without my splendid teammates in the U.K. and Germany.
“We’ll keep on investigating atoms connected with crocagins that probably share a similar center construction.” “We’re anticipating perceiving the way that different analysts expand on the fascinating prospects we’ve revealed in this paper.”
More information: Sebastian Adam et al, Unusual peptide-binding proteins guide pyrroloindoline alkaloid formation in crocagin biosynthesis, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01153-w