Another paper in Nature Correspondences enlightens how a formerly ineffectively comprehended catalyst functions in the cell. Numerous illnesses are attached to ongoing cell stress, and UMBC’s Aaron T. Smith and partners found that this catalyst assumes a significant part in the cell stress reaction. A better understanding of how this compound functions and is controlled could prompt the revelation of new restorative focuses for these illnesses.
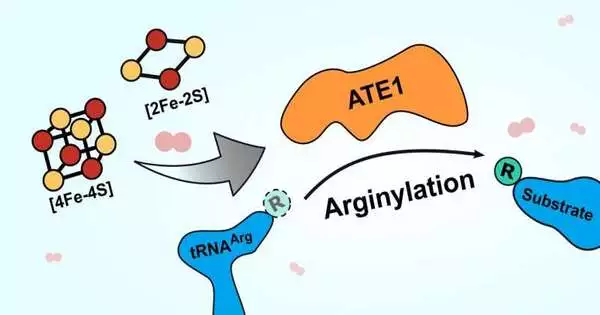
The protein is named ATE1, and it has a place in a group of compounds called arginyl-tRNA transferases. These chemicals add arginine (an amino corrosive) to proteins, which frequently causes the proteins’ obliteration in the cell. Obliterating proteins that are misfolded, frequently because of cell stress, is essential to keep those proteins from unleashing devastation with cell capability. A gathering of failing proteins can create difficult issues in the body, prompting sicknesses like Alzheimer’s or disease, so having the option to dispose of these proteins effectively is critical to long-term wellbeing.
Enticing ramifications
The new paper demonstrates that ATE1 ties to bunches of iron and sulfur particles and that the catalyst’s action increases by a few folds when it is bound to one of these iron-sulfur groups. Additionally, when the specialists impeded cells’ capacity to deliver the groups, ATE1 movement diminished emphatically. They likewise observed that ATE1 is profoundly delicate to oxygen, which they accept connects with its part in directing the cell’s pressure reaction through a cycle known as oxidative pressure.
“Since the yeast protein and the mouse protein behave similarly, there’s reason to expect that the human protein, which is relatively similar to the mouse protein, will likely behave similarly as well.”
Aaron T. Smith
“We were extremely amped up for that, since it has loads of bright, tempting downstream ramifications,” especially connected with the compound’s part in sickness, says Smith, an academic administrator of science and organic chemistry.
Smith’s lab worked at first with the yeast protein, yet additional studies showed that the mouse variant of ATE1 acts in basically the same manner. Smith believes this is significant.”Since the yeast protein and the mouse protein act the same way,” he says, “there’s motivation to accept that on the grounds that the human protein is very similar to the mouse protein, it probably acts the same way also.”
Another methodology
Before they made their leading-edge disclosure, Smith and his graduate understudy Verna Van, Ph.D. ’22, of organic chemistry and sub-atomic science, had been endeavoring for a long time to prompt ATE1 to tie with heme, a compound that contains iron and is important to tie oxygen in blood, to affirm another gathering’s outcomes. It wasn’t working, and they were getting disappointed, Smith concedes. In any case, at some point, as Smith was setting up a talk on proteins in that tight spot with bunches of metal and sulfur iotas, he understood the proteins he was going to cover with his understudies seemed to be like ATE1.
After that acknowledgment, Smith and Van adopted another strategy. In the lab, they added the unrefined substances for making iron-sulfur bunches to an answer with ATE1, and the outcomes showed that ATE1 did indeed tie the groups. “This looks encouraging,” Smith thought. “We were really amped up for it.”
The way that the compound ties the groups at everything was fascinating and new, “however, at that point we additionally inquired as to whether that is influencing the protein’s capacity to do what it does,” Smith says. The response, after over a time of extra examinations, was a resounding yes. Simultaneously, Smith’s gathering additionally resolved the design of ATE1 in yeast (without the group bound to it), which they distributed in the Diary of Atomic Science in November 2022.
Unobtrusive yet critical
Around a similar time, another gathering likewise distributed a somewhat unique ATE1 structure. The other gathering’s design had a zinc particle (another metal) bound instead of the iron-sulfur group. With the zinc set up, one key amino corrosive is pivoting around 60 degrees. It could appear to be unimportant, but Smith accepts that the revolution, which he assumes is comparative with the bunch, is the way into the group’s part in ATE1’s capability.
The turned amino corrosive is straightforwardly neighboring where a protein would communicate with ATE1 to be changed, at last hailing it for corruption. Changing the point of that amino corrosive changes the state of the area the protein would bind “unobtrusively,” however, it changes its action “more than inconspicuously,” Smith says.
Looking forward and thinking back
Smith might likewise want to investigate how different metals, past zinc and the iron-sulfur bunch, may influence the catalyst’s movement. Furthermore, his lab is attempting to determine the design of ATE1 in a living organism other than yeast and to confirm the ATE1 structure with an iron-sulfur bunch.
This large number of steps will help develop a clearer image of how ATE1 works and is directed in the cell. Smith likewise says he accepts proteins that up until this point have not been displayed to tie iron-sulfur groups may, to be sure, depend on them.
This new paper really harkens back to Smith’s most memorable days at UMBC. He has always been keen on making protein changes, and adding arginine is a more surprising oneeen on making protein changes, and adding arginine is a more surprising one. “It’s continuously something that I had recorded back in my brain, and I thought, ‘Gracious, it would be truly fascinating to get a superior comprehension of how that functions,'” he says.
Quite a while later, his gathering is currently on the cutting edge of finding how arginine changes impact cell capability and illness.
More information: Verna Van et al, Iron-sulfur clusters are involved in post-translational arginylation, Nature Communications (2023). DOI: 10.1038/s41467-023-36158-z