New studies conducted by a group of researchers at the Cornell University Center for Bright Beams have made important advancements in the development of novel methods to direct the growth of materials used in next-generation particle accelerators.
The research, which was published in the Journal of Physical Chemistry C, shows that it may be possible to exert more control over the development of superconducting Nb3Sn films, which could significantly reduce the price and size of cryogenic infrastructure needed for superconducting technology.
Niobium superconducting radio frequency (SRF) cavities are used to produce high-energy beams in superconducting accelerator facilities, such as those used for X-ray free-electron laser radiation. However, the associated cryogenic infrastructure, energy requirements, and operating expenses of niobium SRF cavities restrict the use of this technology.
“Advancing this science requires diverse collaborations, which is an important focus at the heart of CBB.” The skills and strong partnerships of all of our partner universities are propelling this study forward.”
Ritchie Patterson, the Helen T. Edwards Professor of Physics in the College of Arts and Sciences.
In order to solve this problem, scientists have been searching for superconducting substances with properties similar to those of niobium (Nb) SRF cavities and operating at temperatures higher than 2 Kelvin. Triniobium tin (Nb3Sn), an alloy with an operating temperature of 18 Kelvin that eliminates the need for pricey cryogenic infrastructure, is one of the most promising materials.
There is still a need for a thorough understanding of how to grow higher quality Nb3Sn alloy films despite theoretical and experimental advances in the performance of Nb3Sn-coated cavities.
According to Ritchie Patterson, the Helen T. Hughes Professor of Physics, “Nb3Sn cavities will be the accelerators of the future.”. Center for Bright Beams director and Edwards Professor of Physics in the College of Arts and Sciences. “Diverse collaborations are essential to advancing this science, and CBB places a high priority on these partnerships. Future directions of this research are being driven by the knowledge and close working relationships among all of our partner institutions. “.
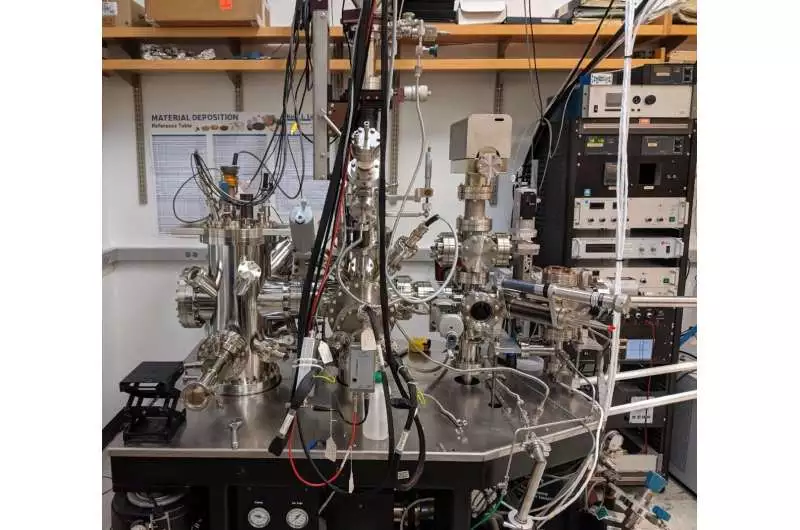
The metal deposition chamber. Credit: Cornell University
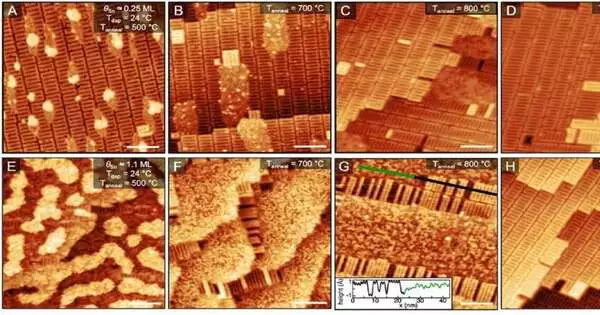
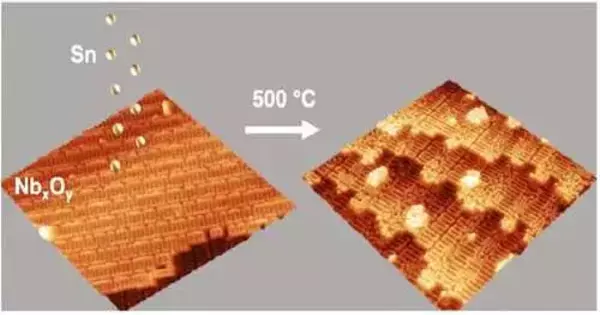
The University of Florida’s theoretical physicists and the University of Chicago’s experimental materials chemists collaborated on this new CBB study to produce the first atomic-scale images of Sn on oxidized niobium that show the beginning stages of Nb3Sn formation. An important step toward developing a mechanistic formula for maximizing the fabrication of next-generation accelerator cavities is the visualization of Sn adsorption and diffusion on oxidized niobium.
The quality and accelerating performance of Nb3Sn are dependent on a number of complex factors that are active during the growth process, according to Sarah Willson, a graduate student in CBB at the University of Chicago and co-author of the paper with postdoctoral researcher Rachael Farber. “We want to examine the first stages of a challenging growth process and isolate specific variables in a regulated environment. The quantum theory developed by graduate student Ajinkya Hire supports their atomic-level growth experiments.
In order to achieve a cleaner and more uniform surface, researchers strive to reduce impurities and contaminants from the niobium cavity during the preparation of Nb3Sn accelerator cavities. After that, a Sn vapor is present while the cavity is heated to a high temperature. As a result, the Sn diffuses into the Nb layer and forms Nb3Sn. Looking closely across the cavity while taking care to grow a flawless Nb3Sn film reveals a highly disordered, rough, polycrystalline surface—not the consistent single-crystal surface ideal for a strictly controlled experiment.
Willson explains that in order to carry out this experiment, they somewhat mimic the actual process of creating a cavity while exceeding the necessary temperature requirements. They heat the materials to 1630 degrees Celsius and produce an atomically flat niobium oxide surface to demonstrate the interactions of Sn, Nb, and O at the atomic level.
Scanning tunneling microscopy, or STM, is frequently used to observe metal oxides and reveals data at the atomic level. However, the precise setup for using STM to study Nb3Sn growth is not easily accessible. Therefore, one was made by Willson and Farber.
To deposit the Sn on the niobium surface, they created and built a special metal deposition chamber. With the capacity to avoid surface contamination, this method simulates the actual environment in which accelerator cavities develop while enabling researchers to study the deposition using STM.
Credit: The Journal of Physical Chemistry C (2023). DOI: 10.1021/acs.jpcc.2c08458
“We have added the intermetallic growth chamber that allows us to do these experiments in-situ,” explains Willson, adding that using the intermetallic growth section reveals the individual Sn atoms integrating with the niobium subsurface. The state-of-the-art STM setup was not really built to study high temperature metallic growth and alloy formation.
“We see that even in our highly controlled environment, the Nb surface serves as a major roadblock in preventing Sn diffusion required for Nb3Sn formation,” says Willson. More than just creating a uniform coating layer of tin on niobium is required to improve Nb3Sn growth, according to the researcher. “.
Alumnus Professor of Materials Science and Engineering at the University of Florida and CBB faculty member Richard Hennig worked with Steven Sibener, the study’s corresponding author, to conduct this research. Steven Sibener is the Carl William Eisendrath Distinguished Service Professor at the University of Chicago.
Sibener, a physical chemist, claims that the collaboration between various accelerator and non-accelerator sciences is exceptional in his experience, laying the foundation for improving particle accelerators, and he looks forward to the promising developments of Nb3Sn.
“This research has definitely been empowered and strengthened by the collaborations that CBB sparks, the ability for surface chemists, materials engineers, accelerator physicists, and theorists to interact in this way,” says Willson. “I personally developed a deeper understanding of how to successfully navigate the difficulties brought on by the various jargons, priorities, and research perspectives across scientific fields. These interfacial metallic growth difficulties that engineers and physicists face pique the interest of many chemists. Conducting a study like this was made easier and more productive by the collaboration, which enabled extensive interdisciplinary communication. “.
More information: Sarah A. Willson et al, Submonolayer and Monolayer Sn Adsorption and Diffusion Behavior on Oxidized Nb(100), The Journal of Physical Chemistry C (2023). DOI: 10.1021/acs.jpcc.2c08458