Alcohol is made, for instance, by microorganisms using CO2 and electricity in microbial electrosynthesis. However, up until this point, it has only been hypothesized how this process functions biologically. It has now been demonstrated experimentally for the first time by researchers at the Leibniz Institute for Natural Product Research and Infection Biology (Leibniz-HKI) that the bacteria can produce more chemicals than previously thought by utilizing electrons from hydrogen. Their exploration has been published in the journal Green Science.
A promising technology in light of climate change and the energy transition is microbial electrosynthesis. It has the ability to bind carbon dioxide, make ethanol and other organic compounds that can be used as fuel, and store excess electricity. In any case, the innovation, which has been known for over 10 years, has so far neglected to accomplish any huge leap towards commercialization.
This is primarily due to the fact that “the biology behind the process has thus far been regarded as a kind of black box,” as stated by Miriam Rosenbaum, head of the Bio Pilot Plant at Leibniz-HKI. The biochemist at Friedrich Schiller University in Jena, who holds the Chair of Synthetic Biotechnology, has long been interested in the specifics of microbial electrosynthesis (MES).
“Amino compounds are very interesting for the chemical industry, and the bacteria we used are already in use in industry.” We may have identified a new method of producing such compounds as a result.”
Santiago Boto, lead author of the study.
In precisely this area, the team has recently made a breakthrough. The researchers were successful in demonstrating that bacteria transfer electrons through hydrogen rather than directly absorbing them. This had been a possibility for a long time, but no one had provided experimental evidence up until this point. Additionally, they optimized the procedure to achieve the highest yields possible and discovered that the method could produce even more useful chemicals than previously thought.
Controlled conditions
In MES, power is applied to a fluid supplement arrangement containing microorganisms, and carbon dioxide is added simultaneously. The carbon and electricity are used by the microorganisms to produce organic compounds like ethanol and acetate. They use the provided electrons to accomplish this, but it was previously unknown how.
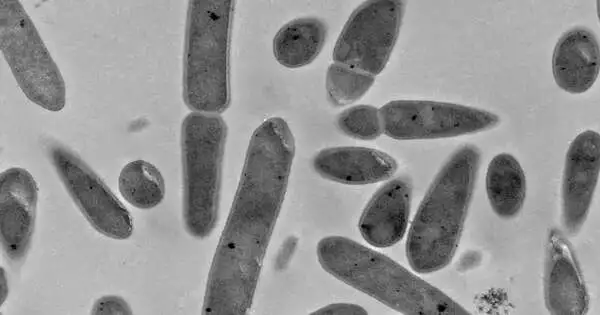
Image of the bacterium Clostridium ljungdahlii taken with an electron microscope. Credit: Sara Al Sbei/Leibniz-HKI and Martin Westermann/ EMZ Jena
“There was one study that assumed that the microbes used the electrons directly,” according to Rosenbaum. This hypothesis, however, was not supported. Rosenbaum believed that the microbes were more likely to use hydrogen for biosynthesis. This is because the process that occurs when carbon dioxide and electricity are combined is identical to that of conventional electrolysis: hydrogen and oxygen are separated from water.
According to Santiago Boto, the study’s lead author, “no one has really measured hydrogen directly in the system up until now.” As a result, the MES reactor was set up so that he could precisely control all of the parameters. He uses a pure culture of the bacterium Clostridium ljungdahlii at various concentrations to accomplish this. He can also control the flow of the electric current and use microsensors to measure the hydrogen produced at the electrode and the hydrogen released from the liquid.
Boto stated, “We were able to gather several pieces of evidence that the bacteria were using hydrogen. This was made possible by our design.” The activity of the bacteria was significantly reduced when the concentration of bacteria in the nutrient medium was such that they formed a biofilm on the cathode and little hydrogen was detected in the electrode environment. When the voltage was too low for electrolysis, this also happened. Just when hydrogen was openly free to plankton— for example, during free swimming—the electrode-borne bacteria displayed high activity.
Discovered new biosynthetic pathways
In this way, the researchers were able to maximize acetate yields by adjusting voltage and bacterial concentration. “For a pure culture of bacteria, we achieved the highest acetate values to date,” Boto stated. He also discovered the formation of amino compounds that the bacteria normally do not produce as a side effect. In collaboration with Falk Harnisch from the Environmental Research Center in Leipzig, the work also demonstrated that the synthesis process appears to be accelerated by reactions between the nutrient medium and the cathode that had not previously been described.
The team now intends to specifically investigate the previous findings and further optimize the processes. The bacteria we used already have applications in industry, and amino compounds are very interesting for the chemical industry. As a result, we may have discovered a novel method of producing such chemicals,” Boto stated.
Overall, the findings ought to contribute to the commercial viability of MES. “When we finally focus on biology as well, I expect that we will see a strong upswing in this technology in the coming years,” Rosenbaum stated. In order to develop larger reactors for MES, the Bio Pilot Plant and process engineers are working together on this.
More information: Santiago T. Boto et al, Microbial electrosynthesis with Clostridium ljungdahlii benefits from hydrogen electron mediation and permits a greater variety of products, Green Chemistry (2023). DOI: 10.1039/D3GC00471F