Researchers have witnessed the mesmerizing process of nanoparticles self-assembling into solid materials for the first time ever. In the stunning new videos, particles fall, tumble down stairs, and slide around before finally snapping into place to create the characteristic stacked layers of a crystal.
The research team, led by Northwestern University and the University of Illinois at Urbana-Champaign, claims that these fresh perceptions could be used to design new materials, including thin films for electronic applications.
In the journal Nature Nanotechnology, the study will be released on March 30.
The study, which the authors dubbed an “experimental tour de force,” used a newly optimized type of liquid-phase TEM to gain previously unattainable insights into the self-assembly process. Prior to this research, scientists used microscopy to observe colloids that were 10 to 100 times larger than nanoparticles, or micron-sized, self-assemble into crystals. To see individual atom layers in a crystalline lattice, they have also used electron or X-ray crystallography. However, they were unable to see individual atoms move into position.
“We can see it coming together right before our eyes now. When we look at nanoparticles, we are looking at particles that are larger than atoms but smaller than colloids. So we’ve covered the entire range of length scales. We are making up the difference in length.”
Northwestern’s Erik Luijten, who led the theoretical and computational work to explain the observations.
Theoretical and computational work to explain the observations was led by Erik Luijten of Northwestern University. “We know that atoms use a similar scheme to assemble into crystals, but we have never seen the actual growth process,” he said. “At this point, we can see it coming together in front of our eyes. Particles that are bigger than atoms but smaller than colloids are what we are watching when we observe nanoparticles. As a result, the full range of length scales has been covered. We are adding the omitted length.”.
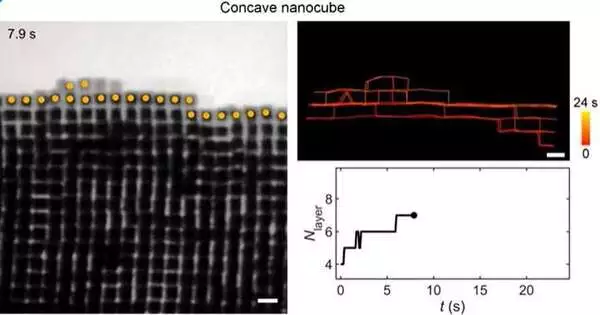
Layer-by-layer growth of a crystal with a smooth surface from gold concave nanocubes is shown in this liquid-phase TEM video. The growing crystal’s surface particles are tracked (the center positions are overlaid with yellow dots).
“Our team previously solved the mystery of nucleation, namely how the embryos of crystals composed of tens of nanoparticles are formed, which follows a nonclassical pathway in solution,” said Qian Chen from the University of Illinois, who oversaw the experimental work. We are now able to record and follow the motions of thousands of nanoparticles over time thanks to recent developments in liquid-phase TEM and data science, as stated in this work. These nanoparticles move around in solutions and develop into crystals with different morphologies, such as polyhedral or wedding cake crystals.
At Northwestern’s McCormick School of Engineering, Luijten teaches materials science and engineering and serves as an associate dean. At Illinois, Chen teaches materials science and engineering as an associate professor.
The majority of people are familiar with crystals in the shapes of salt, sugar, snowflakes, and sparkling gems like diamonds. Although crystallization is a common occurrence, it is still unclear how crystals are created. The fundamental units—atoms, molecules, or ions—that make up crystalline materials are highly ordered and arrange themselves into lattices with uniform spacing. The resulting three-dimensional solid material is created by stacking these lattices on top of one another.
Crystals have smooth, flat faces because their atoms are arranged in regular stacks, according to Luijten. They crack along straight edges because of this.
Researchers have previously examined much larger particles known as colloids to study crystallization. However, observing colloids self-organize into crystals did not provide any new information about how atoms behave. The surfaces of crystals are flat and uniform, whereas the surfaces of crystalline structures made from micron-sized colloids are typically non-uniform and rough.
It is unlikely that colloids crystallize using the same process as atoms because of their size, according to Luijten. Therefore, they don’t instruct us on what atoms do. It is not really accurate to compare colloids to atoms.”.
An animation showing the growth modes for gold concave nanocube crystals in a liquid-phase transmission electron microscope (TEM) chamber.
Luijten, Chen, and their teams used nanoparticles to gain a deeper understanding of the crystallization process. Nanoparticles can now be observed in real time as they solidify thanks to recent improvements to liquid-phase TEM. To ensure that the electron beam could view the particles without causing them harm, Chen’s team spent years refining the procedure. To examine how shape affects behavior, the researchers in the new study used nanoparticles with various shapes, including cubes, spheres, and indented cubes.
Advanced computer simulations, carried out by postdoctoral fellow Tine Curk, graduate students Ziwei Wang and Garrett Watson from Northwestern, and by the researchers themselves, were used to first visualize the formation of crystals. They then carried out experiments with liquid-phase TEM to observe the nanoparticles’ self-assembly in real time. The particles impacted one another during the experiments, sticking together to form layers as a result. The particles first created a horizontal layer, which was followed by a vertical layer to create the layer-by-layer crystalline structure. Occasionally, after adhering to one another, the particles will briefly separate and fall onto a layer beneath.
They run along, pause at the edge, and then fall, according to Luijten. It’s like a diver who is hesitant to jump off a diving board. The fact that we can actually see this is incredible. We have never before observed the growth process itself; only the outcome.”.
Engineers can create new materials with the help of this information, according to Luijten. With regard to thin-film materials, which are frequently used to create flexible electronics, light-emitting diodes, transistors, and solar cells, the insight could be particularly useful in their design.
According to Luijten, “understanding how particles combine will allow us to control the shape of a surface.”. The surface can be affected by changes in particle shape or the rate at which the particles fall.”.
More information: Erik Luijten, Unravelling crystal growth of nanoparticles, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01355-w. www.nature.com/articles/s41565-023-01355-w