Hereditarily speaking, we are people not quite the same as one another in light of slight variations in our DNA groupings—supposedly hereditary variations—some of which have sensational impacts we can see and understand, from the shade of our eyes to our gamble for creating schizophrenia, a crippling mental condition influencing a large number around the world.
For quite a long time, researchers have concentrated on the whole genomes of thousands of individuals—called broad affiliation studies, or GWAS—to find roughly 5,000 hereditary variations related to schizophrenia.
Presently, UNC Institute of Medication researchers and partners are sorting out which of these variations has a causal impact on the improvement of schizophrenia. They are finding that some of the hereditary variations manage or modify the declaration of qualities associated with the condition.
“Our research provides insights into the complex regulatory architecture of genes and proposes a ground-breaking method to decode the cumulative impact of genetic variants on gene regulation in schizophrenia patients,”
Hyejung Won, Ph.D., associate professor of genetics at the UNC School of Medicine.
Distributed in the journal Cell Genomics, this examination denotes a major step in the right direction in how we might interpret the hereditary premise of schizophrenia.
“Our discoveries not only give experiences into the multifaceted administrative scene of qualities, yet in addition propose a weighty way to deal with deciphering the combined impact of hereditary variations on quality guidelines in people with schizophrenia,” said senior creator Hyejung Won, Ph.D., academic administrator of hereditary qualities at the UNC Institute of Medication. “This understanding might actually clear the way for additional exact mediations and treatments later on. In the present moment, helpful choices are restricted, and certain individuals don’t find drugs accessible.”
For this review, Won and first writers Jessica McAfee and Sool Lee, both UNC-Sanctuary Slope graduate understudies, drove a group of scientists from UCLA, Harvard, the College of Michigan, and Human Technopole in Italy to investigate the hereditary variations previously connected to the risk of schizophrenia through GWAS research.
Their objective was to sort out a method for prodding that separated good-for-nothing variations from those with the potential for organic action that was significant for creating schizophrenia. This is difficult for a couple of reasons, one of which is that hereditary variations are frequently acquired from guardians. In this way, right next to one another could be two hereditary variations related to the condition—one may be significant for quality articulation and assume a significant part in the condition, yet the other variation probably won’t play any part in the condition.
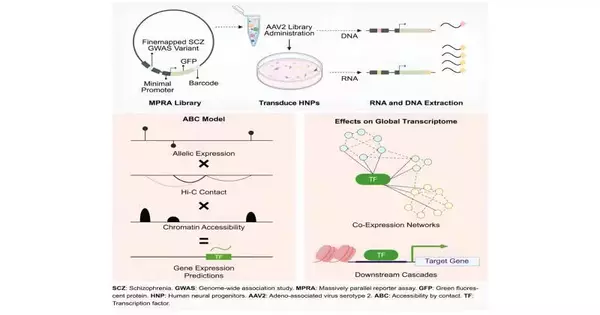
To handle this issue, the scientists utilized an extraordinary procedure called a hugely equal correspondent examination (MPRA)—basically a hereditary sequencing strategy that can parse which variations trigger quality articulation and which ones don’t. To utilize this strategy, the scientists brought the 5,000 variations into human synapses in a dish, cells that are fundamental for early mental health.
These variations might cause the declaration of their downstream quality and hereditary standardized tag. The standardized identification, a 20-bp DNA sequence, is interesting for every variation. This is the thing the gathering uses to recognize the variations in succession. The MPRA uncovered 439 hereditary varieties with genuine natural impacts, meaning they can adjust the articulation of quality.
“Generally, researchers have utilized other epigenetic information, for example, record factor restricting and biochemically characterized enhancers, to recognize variations with organic impacts,” Won said.
“In any case, these ordinary strategies neglected to foresee a huge part of the variations we distinguished to make organic impacts. Our work focuses on an abundance of neglected variations with natural impacts.”
To comprehend how these variations cooperate to impact quality action, Won and partners fostered another model that consolidates information from MPRA with chromatin engineering of synapses—that is, the hereditary data significant for how synapse DNA is coordinated. By doing this, the scientists could interface these 439 variations with how qualities are turned on or off.
“Schizophrenia is a complicated condition that is exceptionally heritable,” Won said. “To find these 439 possibly causal variations is a major step; however, we actually have a ton of work ahead to sort out the muddled hereditary design that drives a person to foster this condition. With that data close by, we could start to comprehend the organic system hidden in this mind-boggling jumble, which may ultimately prompt designated treatments.”
More information: Jessica C. McAfee et al, Systematic investigation of allelic regulatory activity of schizophrenia-associated common variants, Cell Genomics (2023). DOI: 10.1016/j.xgen.2023.100404