Radiation treatment, according to a new study from Washington University School of Medicine in St. Louis, can reprogram cardiac muscle cells to seem younger, correcting electrical abnormalities that cause a life-threatening arrhythmia without the need for an intrusive operation.
A catheter is introduced into the heart during catheter ablation, and the tissue that causes the life-threatening irregular heart rhythm ventricular tachycardia is burnt, leaving scars that block the incorrect impulses.
However, a recent study demonstrates that noninvasive radiation treatment, which is often used to treat cancer, can reprogram cardiac muscle cells to a younger and possibly healthier state, resolving the electrical problem in the cells without the requirement for scar tissue to block the hyperactive circuits.
The study also implies that the same cellular reprogramming effect might be achieved with lower radiation doses, implying that radiation therapy could be used to treat a wider range of cardiac arrhythmias.
The findings were published in the journal Nature Communications on September 24. In 2017, physician-scientists from Washington University demonstrated that radiation therapy, which is generally used to treat cancer, might be used to treat ventricular tachycardia.
Traditionally, catheter ablation creates scar tissue to block the electrical circuits that are causing ventricular tachycardia.
Stacey L. Rentschler
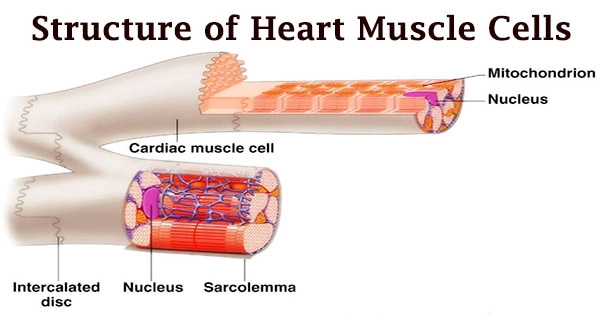
The myocardium, or cardiac muscle tissue, is a specialized kind of muscle tissue that creates the heart. The heart is kept pumping blood around the body by this muscular tissue, which contracts and releases involuntarily. Cardiac muscle is found solely in the heart. It includes cardiac muscle cells, which work together to keep the heart beating and the blood flowing throughout the body.
In principle, radiation therapy might mimic the scar tissue formed by catheter ablation but with a much shorter and less intrusive process, allowing more seriously sick patients to benefit from the treatment.
Surprisingly, the doctors discovered that patients’ arrhythmias improved dramatically within a few days to weeks of radiation therapy, much faster than the months it takes for scar tissue to form after radiation therapy, implying that a single dose of radiation can reduce arrhythmia without forming scar tissue.
For some individuals with ventricular tachycardia, radiation therapy worked equally as well, if not better, than catheter ablation, although in a different and unknown method.
“Traditionally, catheter ablation creates scar tissue to block the electrical circuits that are causing ventricular tachycardia,” said senior author and cardiologist Stacey L. Rentschler, MD, Ph.D., an associate professor of medicine, of developmental biology and of biomedical engineering.
“To help us understand whether the same thing was happening with radiation therapy, some of the first patients to have this new treatment gave us permission to study their heart tissue following heart transplantation or if they had passed away for another reason, for example. We saw that scar tissue alone could not explain the remarkable clinical effects, suggesting that radiation improves the arrhythmia in some other way, so we delved into the details of that.”
Radiation therapy caused cardiac muscle cells to express various genes, according to the researchers. They discovered increased activity in the Notch signaling pathway, which is renowned for its importance in early development, including the formation of the heart’s electrical conduction system.
In mature cardiac muscle cells, Notch is normally turned off. However, the researchers discovered that a single dose of radiation stimulates Notch signaling for a short time, resulting in a long-term increase in sodium ion channels in the heart muscle, a significant physiologic shift that can help to prevent arrhythmias.
“Arrhythmias are associated with slow electrical conduction speeds,” Rentschler said. “Radiation therapy seems to kick up the speed faster by activating early developmental pathways that revert the heart tissue back into a healthier state.”
These effects were tested in rats and human hearts that had been donated. The researchers discovered that these alterations in cardiac muscle cells were exclusively apparent in parts of the heart that got the targeted radiation dosage in human heart samples.
“Radiation does cause a type of injury, but it’s different from catheter ablation,” said co-author and radiation oncologist Julie K. Schwarz, MD, Ph.D., a professor of radiation oncology and director of the Cancer Biology Division in the Department of Radiation Oncology.
“As part of the body’s response to that injury, cells in the injured portion of the heart appear to turn on some of these early developmental programs to repair themselves. It’s important to understand how this works because, with that knowledge, we can improve the way we’re treating these patients and then apply it to other diseases.”
The researchers also discovered that the favorable benefits of radiation lasted at least two years in individuals who survived. And, more importantly, scientists were able to show that a lesser amount of radiation had the same impact on mice.
A lower radiation dose might reduce long-term negative effects and allow this sort of therapy to be used for different forms of cardiac arrhythmias. While Notch is a key actor in these impacts, Schwarz points out that it isn’t the only one. Scientists are still looking into how radiation causes cardiac cells to return to a more healthy condition.
Added first author David M. Zhang, an MD/Ph.D. student in Rentschler’s lab: “This was an exciting collaboration not only between basic scientists and clinicians but also cardiologists and radiation oncologists. Historically, radiation oncologists are focused on cancer and try to avoid irradiating the heart, so this study opens up a whole new area of research and collaboration between these two fields.”
This work was supported by the National Institutes of Health (NIH), grant numbers T32 HL134635, T32 GM07200, R01 HL130212, UH3 HL141800, and S10 OD020136. The Cancer Biology Division of the Department of Radiation Oncology at Washington University provided seed money for this work.
Schwarz is the recipient of an AACR-Bristol Meyers Squibb Female Investigator Award and support from the Radiological Society of North America. Rentschler is the recipient of a Career Award for Medical Scientists from the Burroughs Wellcome Fund, as well as money from The Foundation for Barnes-Jewish Hospital.
Clifford Robinson, MD, and Phillip Cuculich, MD, co-authors at Washington University, have filed two institution-owned patents: Noninvasive Imaging and Treatment System for Cardiac Arrhythmias, which relates to overall methods for delivery of cardiac radiation in patients, and System and Method for Determining Segments for Ablation, which relates to the use of cardiac segments for cardiac radiation targeting. They also give consultancy services to Varian, a company that manufactures linear accelerators for the delivery of radiation therapy.