A global group of specialists, led by researchers at the University of Manchester, has fostered a quick and prudent strategy for changing methane, or flammable gas, into fluid methanol at the surrounding temperature and strain. The strategy happens under a nonstop stream over a photograph reactant material, utilizing noticeable light to drive the change.
To assist with seeing how the cycle functions and how specific it is, the analysts utilized neutron dispersion at the VISION instrument at Oak Ridge National Laboratory’s Spallation Neutron Source.
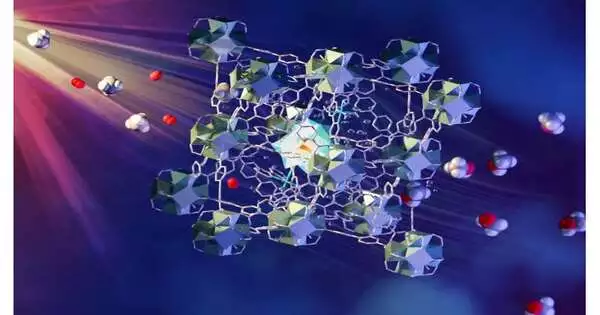
The strategy includes a nonstop progression of methane/oxygen-soaked water over a clever metal-natural system (MOF) impetus. The MOF is permeable and contains various parts that each play a part in retaining light, moving electrons and enacting and uniting methane and oxygen. The methanol is handily removed from the water. Such a cycle has usually been thought of as “a sacred goal of catalysis” and is an area of concentration for research upheld by the U.S. Branch of Energy. Subtleties of the group’s discoveries, named “Direct photo oxidation of methane to methanol over a mono-iron hydroxyl site,” are distributed in Nature Materials.
A global group of specialists, driven by researchers at the University of Manchester, has fostered a quick and prudent strategy for changing methane, or petroleum gas, into fluid methanol at the surrounding temperature and strain. The strategy happens under a nonstop stream over a photograph reactant material, utilizing noticeable light to drive the change.
Normally occurring methane is a bountiful and important fuel, utilized for stoves, heaters, water warmers, ovens, cars, and turbines. In any case, methane can likewise be risky because of the trouble of removing, moving, and putting away it.
Methane gas is likewise unsafe to the climate when it is delivered or spills into the air, where it is a strong ozone-harming substance. Driving wellsprings of air methane incorporate petroleum derivative creation and use, spoiling or consuming biomass like wood fires, rural byproducts, landfills, and softening permafrost.
In abundance, methane is usually singed off, or erupted, to lessen its natural effect. In any case, this burning system produces carbon dioxide, which itself is an ozone-harming substance.
Industry has long looked for a prudent and effective method for changing methane into methanol, a profoundly attractive and flexible feedstock used to make various consumer and modern items. This wouldn’t just assist with lessening methane outflows, yet it would likewise give a monetary impetus to do as such.
Methanol is a more flexible carbon source than methane and is a readily movable fluid. It is used to make a wide range of products, including solvents, radiator fluid, and acrylic plastics; engineered textures and strands; glues, paint, and pressed wood; and compound specialists used in pharmaceuticals and agrichemicals.The change of methane into a high-esteem fuel, for example, methanol, is likewise turning out to be more alluring as oil holds wane.
Breaking the bond
An essential test of changing over methane (CH4) to methanol (CH3OH) has been the trouble of debilitating or breaking the carbon-hydrogen (C-H) synthetic bond to embed an oxygen (O) iota to shape a C-OH bond. Regular methane change strategies commonly include two phases: steam improving followed by syngas oxidation, which are energy intensive, expensive, and wasteful as they require high temperatures and tensions.
The quick and prudent methane-to-methanol process created by the exploration group utilizes a multicomponent MOF material and noticeable light to drive the change. A progression of CH4 and O2 soaked water is gone through a layer of the MOF granules while presented to the light. The MOF contains different planned parts that are found and stand firm on fixed footings inside the permeable superstructure. They cooperate to retain light to create electrons which are passed to oxygen and methane inside the pores to shape methanol.
“To enormously work on the cycle, when methane gas is presented to the useful MOF material containing mono-iron-hydroxyl locales, the enacted oxygen particles and energy from the light advance the enactment of the C-H bond in methane to shape methanol,” said Sihai Yang, a teacher of science at Manchester and a contributing writer. “The cycle is 100 percent specific—meaning there is no unwanted result—similar to methane monooxygenase, which is the protein in nature for this cycle.”
The tests showed the way that the strong impetus can be secluded, washed, dried, and reused for no less than 10 cycles, or around 200 hours of response time, with no deficiency of execution.
The new photocatalytic process is similar to how plants convert light energy to compound energy during photosynthesis. Plants retain daylight and carbon dioxide through their leaves. A photocatalytic cycle then changes these components into sugars, oxygen and water fume.
“This cycle has been named the’sacred goal of catalysis.’ Instead of consuming methane, it might now be feasible to change the gas straightforwardly to methanol, a high-esteem compound that can be utilized to create biofuels, solvents, pesticides, and fuel-added substances for vehicles, “said Martin Schröder, VP and dignitary of staff of science and design at Manchester and the relating creator. “This new MOF material may likewise be suitable for working with different kinds of compound responses by filling in as a kind of test tube in which we can join various substances to perceive how they respond.”
Utilizing neutrons to picture the cycle
“Utilizing neutron dispersing to take ‘pictures’ at the VISION instrument at first affirmed areas of strength for CH4 and the mono-iron-hydroxyl locales in the MOF that debilitate the C-H security,” said Yongqiang Cheng, instrument researcher at the ORNL Neutron Sciences Directorate.
“VISION is a high-throughput neutron vibrational spectrometer improved to give data about sub-atomic design, compound holding, and intermolecular connections,” said Anibal “Timmy” Ramirez Cuesta, who drives the Chemical Spectroscopy Group at SNS. “Methane atoms produce solid and trademark neutron dispersing signals from their rotation and vibration, which are likewise delicate to the nearby climate.” This empowers us to uncover unambiguously the bond-debilitating connections between CH4 and the MOF with cutting-edge neutron spectroscopy methods. “
Quick, prudent, and reusable
By killing the requirement for high temperatures or tensions, and utilizing the energy from daylight to drive the photo oxidation process, the new change strategy could considerably bring down gear and working expenses. The higher speed of the cycle and its capacity to switch methane over completely to methanol with no unwanted results will work with the advancement of in-line handling that limits costs.
More information: Sihai Yang, Direct photo-oxidation of methane to methanol over a mono-iron hydroxyl site, Nature Materials (2022). DOI: 10.1038/s41563-022-01279-1. www.nature.com/articles/s41563-022-01279-1