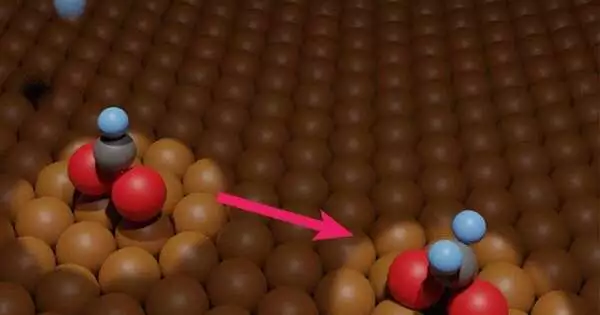
Carbon dioxide contamination keeps on changing the worldwide environment. Scientists know how to pinpoint such contamination, even on a local and close-to-constant premise. As a feature of an answer to carbon dioxide contamination, many examinations center around how to change this poison into a fuel, like methanol. Copper-based impetuses are a device for such changes. Understanding the related bit by bit science is fundamental for improving the change of carbon dioxide toxin into methanol fuel. Nonetheless, the subtleties of this science stay hazy; tests are expected to test speculations that are as of now founded on virtual experiences.
Presently, in a concentrate as of late distributed in the Journal of the American Chemical Society, scientists from the University of Tsukuba and teaming up accomplices have tentatively estimated the hydrogenation of copper-adsorbed formate. This study will assist analysts with enhancing basic strides in the previously mentioned poison-to-fuel cycle and hence speed up methanol creation.
“Hydrogenation of carbon dioxide into methanol is a likely key innovation for creating fuel and compound feedstocks, yet upgrading the response stays troublesome,” makes sense to Dr. Kotaro Takeyasu, senior creator. “That is on the grounds that it’s hard to identify compound intermediates in the bit-by-bit response system tentatively.”
Two primary discoveries were made: infrared reflection retention spectroscopy and temperature-modified desorption. To start with, at a temperature of 200 kelvin, openness to nuclear hydrogen is related to hydrogenation of adsorbed formate. The specific compound nature of the item isn’t yet clear. It was likewise seen that, at a temperature of 250 Kelvin, the hydrogenated formate decayed once more into adsorbed formate or vaporous formaldehyde, in a 96:4 proportion.
“Based on our trial and computational work, the enactment energy of the hydrogenation of adsorbed formate is around 121 kilojoules per mole,” states Dr. Takeyasu. “Our outcomes are steady with the revealed aftereffects of methanol blend studies.”
Copper-zinc amalgams are especially common in this profession. The exploration group is as of now examining how the actuation energies revealed in the current review contrast with especially helpful reactant amalgams, which likewise require trial and computational examinations.
The results of this study will assist analysts with advancing methanol creation from carbon dioxide. Such work will assist with changing an air toxin into fuel for vehicles and compound feedstocks for industry. It gives a method for enhancing carbon dioxide, which is usually viewed as waste. By enhancing the hydrogenation response depicted here, scientists could have another device for utilizing restricted assets.
More information: Kotaro Takeyasu et al, Hydrogenation of Formate Species Using Atomic Hydrogen on a Cu(111) Model Catalyst, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c02797