In science, it has been seen that hereditary data streams from genomic DNA to courier RNA and, lastly, to the protein. The hereditary data inserted inside the DNA has been promoted as the “Language of Life”.
In a cycle called RNA altering, a phone can alter its “draft” message, which is known as a pre-mRNA (before it is handled into a developed courier RNA or mRNA), to change the first trio code (or codon) of the hereditary data of the genome.
This change of the codon utilizing RNA altering can bring about the fuse of an alternate amino corrosive into the protein that hence modify and develop the protein’s capability.
In their most recent paper distributed in the journal, Proceedings of the National Academy of Sciences (PNAS), the group led by Professor Soong Tuck Wah from the Department of Physiology at the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine), has found that the deficiency of RNA altering of the CaV1.3 station has suddenly improved spatial learning and memory through tests.
The group has resolved the changing oddity of this component by easing back the conclusion of the channels, which is the most common way of altering the calcium particle (Ca2+) floods through the channels into the neurons.
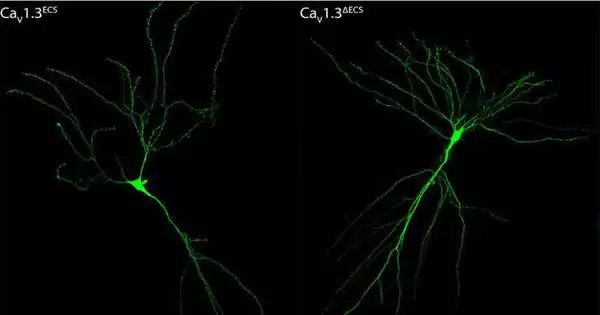
However, RNA modification prevents the CaV1.3 channels from opening, reducing Ca2+ flood.
The unedited CaV1.3 channel permitted more Ca2+ flooding. This end has significant ramifications as the calcium particle flood brought about upgraded spatial memory in the lab model.
As RNA altering isn’t designed in that frame of mind, as seen in hereditary changes, the group had the option to show the way that the degrees of RNA altering can be changed, like changing the splendor of a light by darkening the light.
In this paper, the group showed that CaV1.3 RNA alteration is movable and can influence learning abilities and memory. In view of the tests, the group likewise noticed an entrancing revelation that has to do with the compromises of improved memory and learning. The compromises incorporate tension, weight and rest aggravation.
The group is right now exploring the physiological effects of CaV1.3 RNA altering and a desire to cover their discoveries soon.
As a group objective, the group means to comprehend how CaV1.3 RNA altering directs circadian mood and rest and how rest unsettling influence adds to metabolic turmoil like stoutness, and whether or not social or dietary mediations will ease weight gain or work on metabolic or emotional wellness.
A similar group led by Prof Soong was the first on the planet to discover RNA alteration of the CaV1.3 channel a long time ago.
CaV1.3 channels structure “electrical switches” on the cell surface to direct the flood of Ca2+ particles into the cells. The group published their most memorable paper on this disclosure in one of the top neuroscience journals, Neuron, in 2012.
Since then, they have continued in this manner to focus on the RNA structures in greater depth, in order to comprehend how these designs support the altering of the pre-mRNA. They found that CaV1.3 diverts are specifically altered in synapses known as neurons, yet the CaV1.3 channels are not altered when communicated in fringe organs or tissues like the heart, lung, kidney, or testis. These discoveries were accounted for in Nucleic Acid Research in 2018.
More information: Jing Zhai et al, Loss of Ca V 1.3 RNA editing enhances mouse hippocampal plasticity, learning, and memory, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2203883119
Journal information: Nucleic Acid Research