Scientists in Japan have revealed the system for how the measles infection can cause subacute sclerosing pan encephalitis, or SSPE, an uncommon yet deadly neurological issue that can happen quite a while after a measles disease.
Although the typical type of measles infection can’t taint the sensory system, the group found that infections that endure in the body can foster changes in a key protein that controls how they contaminate cells. The changed proteins can connect with its not unexpected structure, making it fit for tainting the mind. Their discoveries were accounted for in the journal Science Advances.
In the event that you are of a specific age, you might have gotten the measles as a kid. Many brought into the world after the 1970s have never gotten it because of antibodies. The condition is brought about by an infection with a similar name, which is one of the most infectious microbes right now. According to the World Health Organization, nearly 9,000,000 people worldwide were infected with measles in 2021, with 128,000 deaths.
“Despite its availability, the recent COVID-19 epidemic has slowed immunizations, particularly in the developing world. The measles virus causes SSPE, a rare but lethal illness. However, because the typical measles virus cannot replicate in the brain, it is unclear how it produces encephalitis.”
Yuta Shirogane, assistant professor at Kyushu University’s Faculty of Medical Sciences.
“In spite of its accessibility, the new coronavirus pandemic has impaired immunizations, particularly in the Worldwide South,” makes sense to Yuta Shirogane, an aide teacher at Kyushu College’s Staff of Clinical Sciences. “SSPE is an uncommon yet deadly condition brought about by the measles infection.” “However, because the typical measles infection does not spread in the brain, the mechanism by which it causes encephalitis is unclear.”
An infection taints cells through a progression of proteins that jut from their surface. Normally, one protein will initially work with the infection to attach to a cell’s surface, then another surface protein will cause a response that gives the infection access to the cell, prompting a disease. Hence, what an infection can or cannot taint can depend intensely on the type of cell.
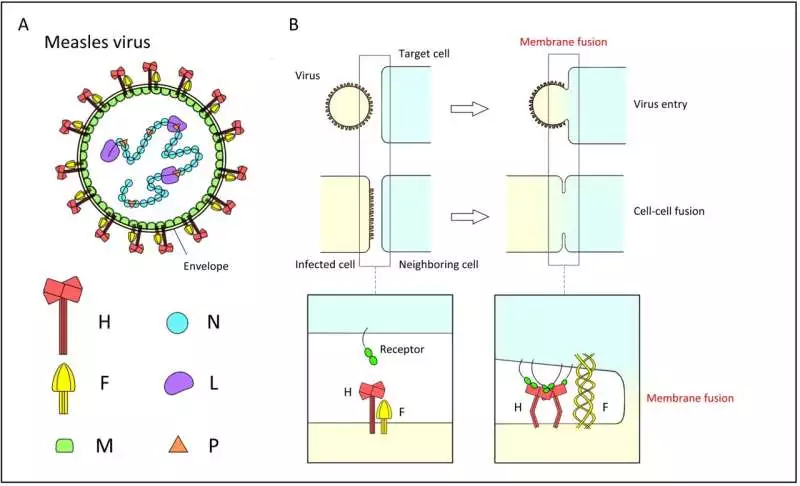
The measles infection is wrapped in a lipid bilayer. The bilayer holds the receptor restricting the hemagglutinin (H) protein and the combination (F) protein. For disease to occur, the H protein first ties to a receptor on the objective cell, and afterward the F protein changes its conformity to meld the layers.
“Normally, the measles infection just taints your skin and epithelial cells, causing the fever and rash,” proceeds Shirogane. “Hence, in patients with SSPE, the measles infection probably stayed in their body and changed, then acquired the capacity to taint nerve cells.” “RNA infections like measles change and develop at high rates, yet the system of how it advances to taint neurons has been a secret.”
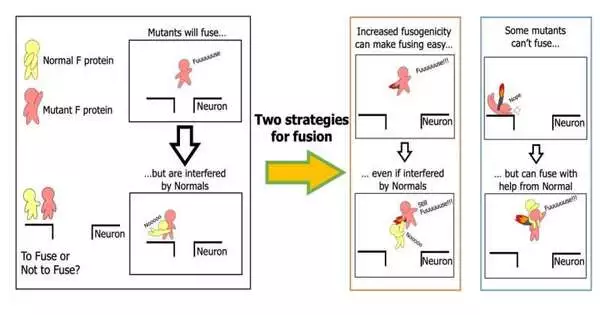
The central component in permitting the measles infection to taint a person is a protein called a combination protein, or F protein. In the group’s past examinations, they showed that specific changes in the F protein place it in a “hyperfusongenic” state, permitting it to meld onto brain neurotransmitters and taint the mind.
In their most recent review, the group examined the genome of the measles infection in SSPE patients and found that different changes had amassed in their F protein. Surprisingly, some changes increased disease activity while others decreased it.
“This was amazing to see, yet we tracked down a clarification.” “When the infection contaminates a neuron, it contaminates it through “en coalition transmission,” in which multiple duplicates of the viral genome enter the cell,” Shirogane continues.”In this case, the genome encoding the freak F protein is sent along with the genome encoding the typical F protein, and the two proteins are likely to coincide in the tainted cell.”
In view of this speculation, the group examined the combination action of freak F proteins when typical F proteins were available. Their outcomes showed that combination action of a freak F protein is stifled because of impedance from the typical F proteins, yet that obstruction is overwhelmed by the gathering of changes in the F protein.
At the point when the F protein incites layer combination at a neuronal neurotransmitter, various measle infection genomes are all the while sent to the following neuron. This peculiarity is known as an ‘en alliance transmission.’ Under this condition, both typical and freak genomes are sent all the while, bringing about ordinary and freak F proteins coexpressed in a tainted cell.
For another situation, the group found that an alternate arrangement of changes in the F protein brought about a totally different outcome: a decrease in combination action. In any case, amazingly, this change can really help out typical F proteins to increase combination action. Hence, even freak F proteins that seem, by all accounts, to not be able to taint neurons can, in any case, contaminate the mind.
“It is practically counter to the “natural selection” model of viral spread.” In fact, this peculiarity in which changes meddle and help one another is known as “sociovirology.”It’s yet another idea, yet infections have been seen to connect with one another like a gathering. “It’s a thrilling possibility,” Shirogane says.
The group trusts that their outcomes will assist with creating therapeutics for SSPE as well as explain the normal developmental systems for infections that have comparable disease components to measles, for example, novel COVIDs and herpesviruses.
“There are numerous secrets in the systems by which infections cause illnesses.” Since I was a clinical understudy, I was keen on how the measles infection caused SSPE. “I’m glad we had the opportunity to clarify the system of this illness,” Shirogane concludes.
More information: Yuta Shirogane, Collective Fusion Activity Determines Neurotropism of an en Bloc Transmitted Enveloped Virus, Science Advances (2023). DOI: 10.1126/sciadv.adf3731. www.science.org/doi/10.1126/sciadv.adf3731